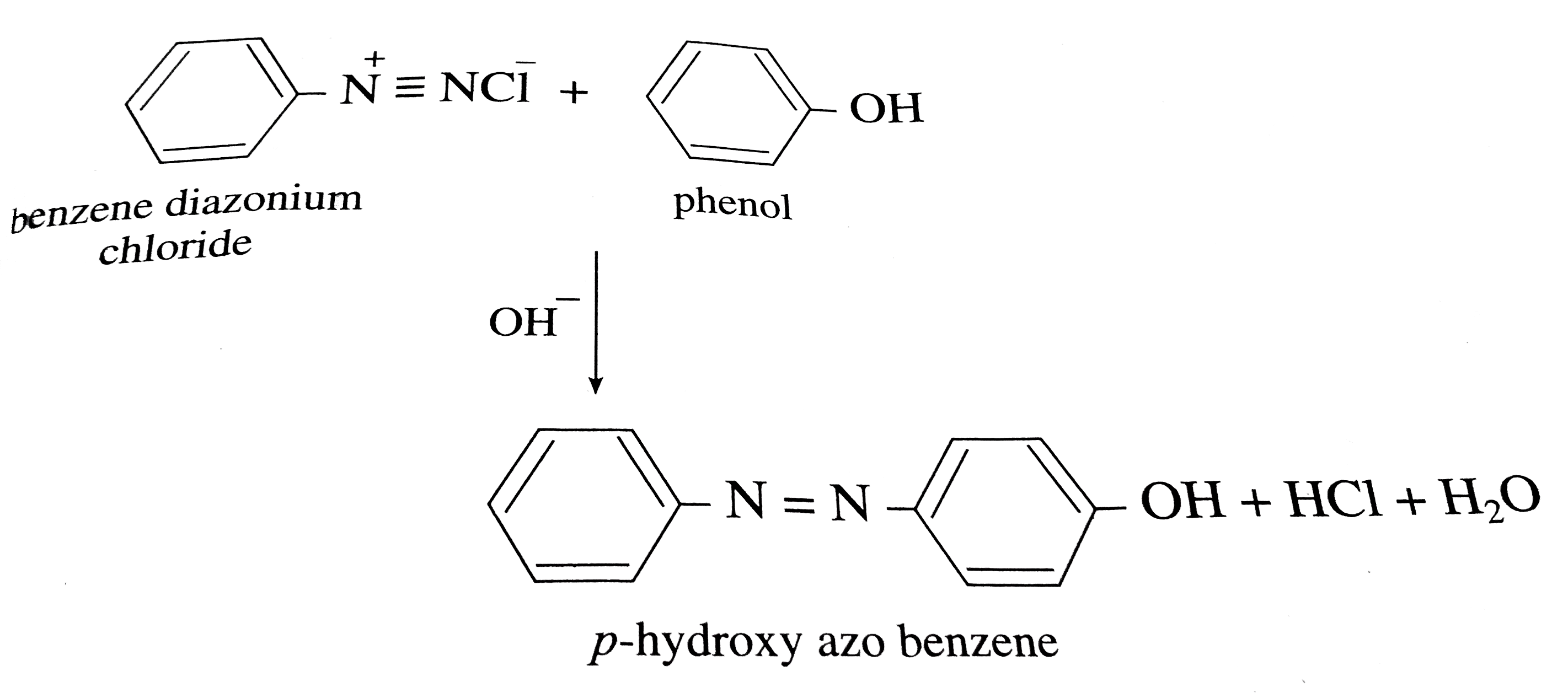
Coupling Of Benzene Diazonium Chloride . Substitution reactions of diazonium ions. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually couple with diazonium cations. Diazonium ions are present in solutions such as benzenediazonium chloride solution. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. This is referred to as a coupling reaction. The general formula is c₆h₅n₂cl. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. It is a type of azo coupling. Diazonium ions are present in solutions such as benzenediazonium chloride solution. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. Substitution reactions of diazonium ions.
from www.doubtnut.com
Diazonium ions are present in solutions such as benzenediazonium chloride solution. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. The general formula is c₆h₅n₂cl. Substitution reactions of diazonium ions. It is a type of azo coupling. This is referred to as a coupling reaction. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. Diazonium ions are present in solutions such as benzenediazonium chloride solution. Substitution reactions of diazonium ions.
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET
Coupling Of Benzene Diazonium Chloride This is referred to as a coupling reaction. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. Substitution reactions of diazonium ions. Diazonium ions are present in solutions such as benzenediazonium chloride solution. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually couple with diazonium cations. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. This is referred to as a coupling reaction. It is a type of azo coupling. Substitution reactions of diazonium ions. Diazonium ions are present in solutions such as benzenediazonium chloride solution. The general formula is c₆h₅n₂cl.
From byjus.com
How to convert benzene diazonium chloride to phenol? Coupling Of Benzene Diazonium Chloride The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually couple with diazonium cations. Diazonium ions are present in solutions such as benzenediazonium chloride solution. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. Diazonium ions are present in solutions. Coupling Of Benzene Diazonium Chloride.
From www.chemca.in
Reaction chart of Benzene diazonium chloride Coupling Of Benzene Diazonium Chloride The general formula is c₆h₅n₂cl. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. Substitution reactions of diazonium ions. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually couple with diazonium cations. Diazonium ions are present. Coupling Of Benzene Diazonium Chloride.
From www.youtube.com
Coupling Reaction of Benzenediazonium Chloride Class 12 Chemistry Coupling Of Benzene Diazonium Chloride It is a type of azo coupling. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. Substitution reactions of diazonium ions. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. Substitution reactions of diazonium ions. The general formula is c₆h₅n₂cl. Aryl diazonium salts may. Coupling Of Benzene Diazonium Chloride.
From byjus.com
59 Explain the mechanism of azo coupling reaction between aniline and Coupling Of Benzene Diazonium Chloride It is a type of azo coupling. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. Diazonium ions are present in solutions such as benzenediazonium chloride solution. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. This is referred to as. Coupling Of Benzene Diazonium Chloride.
From www.toppr.com
Benzene diazonium chloride on reaction with phenol in weakly basic Coupling Of Benzene Diazonium Chloride Substitution reactions of diazonium ions. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. Diazonium ions are present in solutions such as benzenediazonium chloride solution. It is a type of azo coupling.. Coupling Of Benzene Diazonium Chloride.
From www.youtube.com
Coupling reaction of Phenol Phenol Benzene diazonium chloride p Coupling Of Benzene Diazonium Chloride It is a type of azo coupling. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually couple with diazonium cations. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. The general formula is c₆h₅n₂cl. Aryl diazonium salts may be reduced to. Coupling Of Benzene Diazonium Chloride.
From www.youtube.com
Coupling reaction takes place when benzene diazonium chloride is Coupling Of Benzene Diazonium Chloride Diazonium ions are present in solutions such as benzenediazonium chloride solution. This is referred to as a coupling reaction. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually couple with diazonium cations. The general formula is c₆h₅n₂cl. Substitution reactions of diazonium ions. Aryl diazonium salts may be reduced. Coupling Of Benzene Diazonium Chloride.
From www.youtube.com
During coupling reaction of benzene diazonium chloride and aniline Coupling Of Benzene Diazonium Chloride Substitution reactions of diazonium ions. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. Diazonium ions are present in solutions such as benzenediazonium chloride solution. This is referred to as. Coupling Of Benzene Diazonium Chloride.
From www.youtube.com
Coupling of benzene diazonium chloride with 1naphthol in alkaline Coupling Of Benzene Diazonium Chloride It is a type of azo coupling. The general formula is c₆h₅n₂cl. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. The reaction barrier is high however, and it is certainly observed that only activated. Coupling Of Benzene Diazonium Chloride.
From www.toppr.com
Which of the following compounds will not undergo azo coupling reaction Coupling Of Benzene Diazonium Chloride Diazonium ions are present in solutions such as benzenediazonium chloride solution. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. Diazonium ions are present in solutions such as benzenediazonium chloride solution. Substitution reactions of diazonium ions. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium. Coupling Of Benzene Diazonium Chloride.
From www.toppr.com
Write the coupling reaction of benzene diazonium chloride with phenol Coupling Of Benzene Diazonium Chloride For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. It is a type of azo coupling. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. This is referred to as a coupling reaction. The general formula is c₆h₅n₂cl. Aryl diazonium salts may be reduced. Coupling Of Benzene Diazonium Chloride.
From www.vedantu.com
Benzene Diazonium Chloride Learn Important Terms and Concepts Coupling Of Benzene Diazonium Chloride Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. Diazonium ions are present in solutions such as benzenediazonium chloride solution. The reaction barrier is high however, and it is certainly. Coupling Of Benzene Diazonium Chloride.
From www.studypool.com
SOLUTION Chimestry reactions of benzene diazonium chloride Studypool Coupling Of Benzene Diazonium Chloride For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. Diazonium ions are present in solutions such as benzenediazonium chloride solution. It is a type of azo coupling. This is referred to as a coupling reaction. Substitution reactions of diazonium ions. In such reactions diazonium salt shows electrophilic attack on other aromatic. Coupling Of Benzene Diazonium Chloride.
From byjus.com
What happens When Benzene Diazonium Chloride reacts with Naphthol? Give Coupling Of Benzene Diazonium Chloride The general formula is c₆h₅n₂cl. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually. Coupling Of Benzene Diazonium Chloride.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Coupling Of Benzene Diazonium Chloride The general formula is c₆h₅n₂cl. Substitution reactions of diazonium ions. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually couple with diazonium cations. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. Substitution reactions of diazonium. Coupling Of Benzene Diazonium Chloride.
From byjus.com
Which of the following compounds will not undergo azo coupling reaction Coupling Of Benzene Diazonium Chloride For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. The general formula is c₆h₅n₂cl. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. Substitution reactions of diazonium ions. This is referred to as a coupling reaction. It is a. Coupling Of Benzene Diazonium Chloride.
From www.pdfprof.com
benzene diazonium chloride reactions Coupling Of Benzene Diazonium Chloride In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. Diazonium ions are present in solutions such as benzenediazonium chloride solution. The general formula is c₆h₅n₂cl. It is a type of azo coupling. Substitution reactions of diazonium ions. Substitution reactions of diazonium ions. Diazonium ions are present in solutions such as benzenediazonium chloride solution.. Coupling Of Benzene Diazonium Chloride.
From www.doubtnut.com
Coupling of benzene diazonium chloride with 1naphthol in alkaline med Coupling Of Benzene Diazonium Chloride Substitution reactions of diazonium ions. Diazonium ions are present in solutions such as benzenediazonium chloride solution. It is a type of azo coupling. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. Substitution reactions of diazonium ions. For these, the hidden intermediate is stabilized by the substituent, and. Coupling Of Benzene Diazonium Chloride.
From www.pdfprof.com
benzene diazonium chloride reactions Coupling Of Benzene Diazonium Chloride Diazonium ions are present in solutions such as benzenediazonium chloride solution. Substitution reactions of diazonium ions. The general formula is c₆h₅n₂cl. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. Substitution reactions of diazonium ions.. Coupling Of Benzene Diazonium Chloride.
From www.doubtnut.com
Coupling of benzene diazonium chloride with 1naphthol in alkaline med Coupling Of Benzene Diazonium Chloride The general formula is c₆h₅n₂cl. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. Substitution reactions of diazonium ions. Diazonium ions are present in solutions such as benzenediazonium chloride solution. Substitution reactions of diazonium ions. It is a type of azo coupling. The reaction barrier is high however, and it is certainly observed. Coupling Of Benzene Diazonium Chloride.
From www.doubtnut.com
Diazonium salt formation and coupling reaction When a reaction mixtur Coupling Of Benzene Diazonium Chloride For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually couple with diazonium cations. Diazonium ions are present in solutions such as benzenediazonium chloride solution. Diazonium ions are present in solutions. Coupling Of Benzene Diazonium Chloride.
From www.toppr.com
Write the coupling reaction of benzene diazonium chloride with phenol Coupling Of Benzene Diazonium Chloride The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually couple with diazonium cations. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. Substitution. Coupling Of Benzene Diazonium Chloride.
From byjus.com
23. Explain the mechanism of coupling reaction between diazonium ion Coupling Of Benzene Diazonium Chloride Substitution reactions of diazonium ions. This is referred to as a coupling reaction. It is a type of azo coupling. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. The reaction barrier is high however,. Coupling Of Benzene Diazonium Chloride.
From www.toppr.com
Coupling of benzene diazonium chloride with 1 napthol in alkaline Coupling Of Benzene Diazonium Chloride The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually couple with diazonium cations. This is referred to as a coupling reaction. Diazonium ions are present in solutions such as benzenediazonium chloride solution. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as. Coupling Of Benzene Diazonium Chloride.
From www.youtube.com
Coupling reaction takes place when benzene diazonium chloride is tr Coupling Of Benzene Diazonium Chloride Diazonium ions are present in solutions such as benzenediazonium chloride solution. The general formula is c₆h₅n₂cl. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. It is a type of azo coupling. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually. Coupling Of Benzene Diazonium Chloride.
From www.masterorganicchemistry.com
Reactions of Diazonium Salts Sandmeyer and Related Reactions Coupling Of Benzene Diazonium Chloride Diazonium ions are present in solutions such as benzenediazonium chloride solution. The general formula is c₆h₅n₂cl. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. It is a type of azo coupling.. Coupling Of Benzene Diazonium Chloride.
From www.researchgate.net
(a) Synthesis of benzenediazonium chloride and mechanism of Coupling Of Benzene Diazonium Chloride Substitution reactions of diazonium ions. The general formula is c₆h₅n₂cl. Substitution reactions of diazonium ions. It is a type of azo coupling. Diazonium ions are present in solutions such as benzenediazonium chloride solution. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. Diazonium ions are present in solutions. Coupling Of Benzene Diazonium Chloride.
From byjus.com
92/200 Aniline and benzene diazonium chloride arechemically Coupling Of Benzene Diazonium Chloride In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. Diazonium ions are present in solutions such as benzenediazonium chloride solution. The general formula is c₆h₅n₂cl. It is a type of azo coupling. Diazonium ions are present in solutions such as benzenediazonium chloride solution. Substitution reactions of diazonium ions. Substitution reactions of diazonium ions.. Coupling Of Benzene Diazonium Chloride.
From www.vedantu.com
Coupling of benzene diazonium chloride with 1naphthol in alkaline Coupling Of Benzene Diazonium Chloride It is a type of azo coupling. Substitution reactions of diazonium ions. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. Diazonium ions are present in solutions such as benzenediazonium chloride solution. The general formula is c₆h₅n₂cl. Diazonium ions are present in solutions such as benzenediazonium chloride solution. Substitution reactions of. Coupling Of Benzene Diazonium Chloride.
From byjus.com
4. ASSERTION. COMPOUND FORMED BY COUPLING OF BENZENE DIAZONIUM CHLORIDE Coupling Of Benzene Diazonium Chloride Diazonium ions are present in solutions such as benzenediazonium chloride solution. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. Substitution reactions of diazonium ions. Substitution reactions of diazonium ions. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh,. Coupling Of Benzene Diazonium Chloride.
From www.doubtnut.com
Coupling of benzene diazonium chloride with 1naphthol in alkaline med Coupling Of Benzene Diazonium Chloride Diazonium ions are present in solutions such as benzenediazonium chloride solution. Diazonium ions are present in solutions such as benzenediazonium chloride solution. It is a type of azo coupling. This is referred to as a coupling reaction. Substitution reactions of diazonium ions. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh,. Coupling Of Benzene Diazonium Chloride.
From www.toppr.com
What happens when benzene diazonium chloride reacts with phenol in weak Coupling Of Benzene Diazonium Chloride Diazonium ions are present in solutions such as benzenediazonium chloride solution. Substitution reactions of diazonium ions. The general formula is c₆h₅n₂cl. Diazonium ions are present in solutions such as benzenediazonium chloride solution. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. Substitution reactions of diazonium ions. The reaction barrier is high however, and. Coupling Of Benzene Diazonium Chloride.
From www.doubtnut.com
Coupling of benzene diazonium chloride with 1naphthol in alkaline med Coupling Of Benzene Diazonium Chloride Substitution reactions of diazonium ions. Diazonium ions are present in solutions such as benzenediazonium chloride solution. It is a type of azo coupling. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually. Coupling Of Benzene Diazonium Chloride.
From www.doubtnut.com
Coupling of benzene diazonium chloride with 1naphthol in alkaline med Coupling Of Benzene Diazonium Chloride Substitution reactions of diazonium ions. The reaction barrier is high however, and it is certainly observed that only activated arenes (phenols, anilines, x,y=oh, nh 2) actually couple with diazonium cations. Substitution reactions of diazonium ions. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs. Aryl diazonium salts may be reduced to the corresponding. Coupling Of Benzene Diazonium Chloride.
From www.pdfprof.com
benzene diazonium chloride reactions Coupling Of Benzene Diazonium Chloride It is a type of azo coupling. For these, the hidden intermediate is stabilized by the substituent, and no doubt emerges as a real intermediate. This is referred to as a coupling reaction. Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or. Substitution reactions of diazonium ions. In. Coupling Of Benzene Diazonium Chloride.