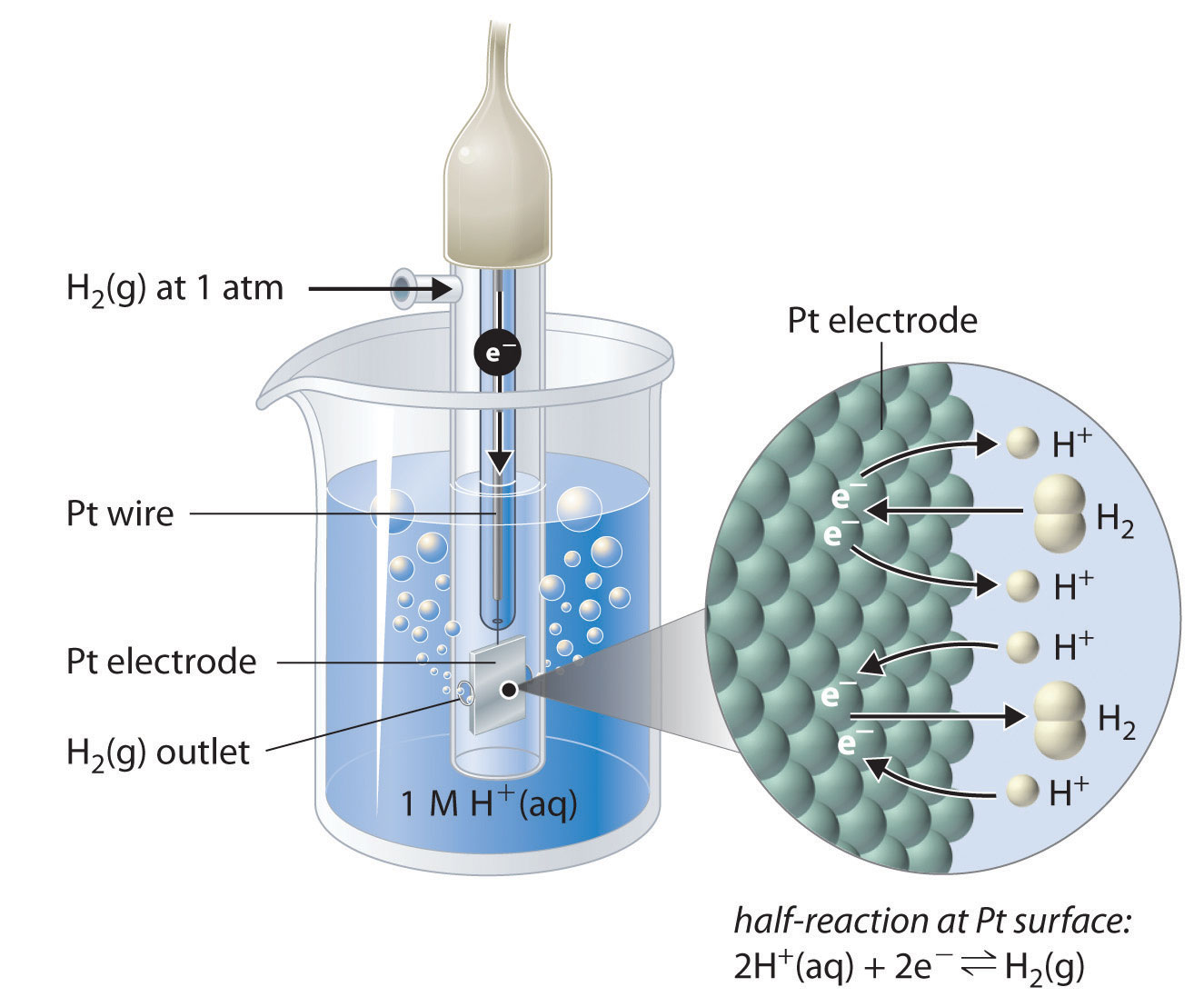
Standard Electrode Potential Gold . 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The iupac gold book defines it. The standard cell potential is a measure of the driving force for a given redox reaction. Reduction reactions in acidic solution are written using h +. All other species are aqueous. Solids, gases, and liquids are identified; 45 rows standard electrode potentials in aqueous solution at 25°c.
from chem.libretexts.org
The standard cell potential is a measure of the driving force for a given redox reaction. All other species are aqueous. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Solids, gases, and liquids are identified; 45 rows standard electrode potentials in aqueous solution at 25°c. Reduction reactions in acidic solution are written using h +. The iupac gold book defines it.
Standard Potentials Chemistry LibreTexts
Standard Electrode Potential Gold 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. The iupac gold book defines it. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard electrode potentials in aqueous solution at 25°c. Reduction reactions in acidic solution are written using h +. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Solids, gases, and liquids are identified; The standard cell potential is a measure of the driving force for a given redox reaction. All other species are aqueous.
From learnah.org
4 Measuring standard electrode potential PAG8 Standard Electrode Potential Gold 45 rows standard electrode potentials in aqueous solution at 25°c. The iupac gold book defines it. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. All other species are aqueous. Solids, gases, and liquids are identified; 372 rows the data below tabulates standard electrode potentials (e°), in. Standard Electrode Potential Gold.
From 2012books.lardbucket.org
Standard Potentials Standard Electrode Potential Gold 45 rows standard electrode potentials in aqueous solution at 25°c. The iupac gold book defines it. Solids, gases, and liquids are identified; Reduction reactions in acidic solution are written using h +. All other species are aqueous. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Gold possesses very. Standard Electrode Potential Gold.
From www.researchgate.net
Electrochemical activity and inert potential range for gold. Download Standard Electrode Potential Gold In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. All other species are aqueous. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative. Standard Electrode Potential Gold.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Redox (II) Standard Electrode Potential Eo (1) Standard Electrode Potential Gold Solids, gases, and liquids are identified; The iupac gold book defines it. 45 rows standard electrode potentials in aqueous solution at 25°c. The standard cell potential is a measure of the driving force for a given redox reaction. All other species are aqueous. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any. Standard Electrode Potential Gold.
From www.chegg.com
Solved 7.17 From the standard electrode potentials in Table Standard Electrode Potential Gold Solids, gases, and liquids are identified; All other species are aqueous. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard electrode potentials in aqueous solution at 25°c. Reduction reactions in acidic solution are written using h +. The standard cell potential is a measure of the. Standard Electrode Potential Gold.
From users.highland.edu
Standard Potentials Standard Electrode Potential Gold 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. All other species are aqueous. The standard cell potential is a measure of the driving force for a given redox reaction. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure. Standard Electrode Potential Gold.
From exovxcvqo.blob.core.windows.net
Standard Reduction Potential Gold at Juan Delk blog Standard Electrode Potential Gold All other species are aqueous. Solids, gases, and liquids are identified; 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Reduction reactions in acidic solution are written using h +. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure. Standard Electrode Potential Gold.
From www.pveducation.org
Standard Potential PVEducation Standard Electrode Potential Gold All other species are aqueous. The iupac gold book defines it. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. Solids, gases, and liquids are identified; The standard cell potential is a measure of the driving force for a given redox reaction. In electrochemistry, standard electrode potential. Standard Electrode Potential Gold.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Electrode Potential Gold The iupac gold book defines it. Reduction reactions in acidic solution are written using h +. Solids, gases, and liquids are identified; The standard cell potential is a measure of the driving force for a given redox reaction. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often.. Standard Electrode Potential Gold.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Electrode Potential Gold The iupac gold book defines it. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. All other species are aqueous. The standard cell potential is a measure of the driving force for a given redox reaction. Reduction reactions in acidic solution are written using h +. 45 rows standard. Standard Electrode Potential Gold.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Standard Electrode Potential Gold Solids, gases, and liquids are identified; Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. The iupac gold book defines it. The standard cell potential is a measure of the driving force for a given redox reaction. All other species are aqueous. 45 rows standard electrode potentials. Standard Electrode Potential Gold.
From pandai.me
Standard Electrode Potential Standard Electrode Potential Gold All other species are aqueous. 45 rows standard electrode potentials in aqueous solution at 25°c. The standard cell potential is a measure of the driving force for a given redox reaction. Reduction reactions in acidic solution are written using h +. Solids, gases, and liquids are identified; 372 rows the data below tabulates standard electrode potentials (e°), in volts relative. Standard Electrode Potential Gold.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Electrode Potential Gold Solids, gases, and liquids are identified; The iupac gold book defines it. 45 rows standard electrode potentials in aqueous solution at 25°c. Reduction reactions in acidic solution are written using h +. All other species are aqueous. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. The. Standard Electrode Potential Gold.
From turtaras.blogspot.com
Astonishing Photos Of Cell Potential Table Concept Turtaras Standard Electrode Potential Gold Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. 45 rows standard electrode potentials in aqueous solution at 25°c. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Reduction reactions in acidic solution are written using. Standard Electrode Potential Gold.
From www.youtube.com
Determination of Standard Electrode potential of 'Zn and Cu Standard Electrode Potential Gold In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. All other species are aqueous. The iupac gold book defines it. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard electrode potentials in aqueous solution at. Standard Electrode Potential Gold.
From mungfali.com
Standard Electrode Potential Table Standard Electrode Potential Gold The standard cell potential is a measure of the driving force for a given redox reaction. The iupac gold book defines it. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Solids, gases, and liquids are identified; Reduction reactions in acidic solution are written using h +. In electrochemistry,. Standard Electrode Potential Gold.
From www.chemistryland.com
Lab 8 Single Replacement Reactions Standard Electrode Potential Gold In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. The standard cell potential is a measure of the driving force for a given redox reaction. 45 rows. Standard Electrode Potential Gold.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Electrode Potential Gold Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. Solids, gases, and liquids are identified; 45 rows standard electrode potentials in aqueous solution at 25°c. All other species are aqueous. The iupac gold book defines it. Reduction reactions in acidic solution are written using h +. In. Standard Electrode Potential Gold.
From www.differencebetween.com
What is the Difference Between Standard Electrode Potential and Standard Electrode Potential Gold In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The iupac gold book defines it. The standard cell potential is a measure of the driving force for a given redox reaction. Reduction reactions in acidic solution are written using h +. 45 rows standard electrode potentials in aqueous solution. Standard Electrode Potential Gold.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Standard Electrode Potential Gold Solids, gases, and liquids are identified; Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The standard cell potential is a measure of the driving force for. Standard Electrode Potential Gold.
From mungfali.com
Table Of Standard Electrode Potentials Standard Electrode Potential Gold Solids, gases, and liquids are identified; 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The standard cell potential is a measure of the driving force for a given redox reaction. The iupac gold book defines it. In electrochemistry, standard electrode potential , or , is a measure of. Standard Electrode Potential Gold.
From www.tpsearchtool.com
Standard Reduction Potential Charts For Chemistry Images Standard Electrode Potential Gold Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. The iupac gold book defines it. Reduction reactions in acidic solution are written using h +. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. All other. Standard Electrode Potential Gold.
From www.sliderbase.com
Electrochemical Terminology Standard Electrode Potential Gold The iupac gold book defines it. Reduction reactions in acidic solution are written using h +. 45 rows standard electrode potentials in aqueous solution at 25°c. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. In electrochemistry, standard electrode potential , or , is a measure of. Standard Electrode Potential Gold.
From www.studypool.com
SOLUTION Standard electrode potential,definition,explanation and Standard Electrode Potential Gold The iupac gold book defines it. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. All other species are aqueous. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. 45 rows standard electrode potentials in aqueous. Standard Electrode Potential Gold.
From askfilo.com
7. Observed and calculated values for the standard electrode potentials o.. Standard Electrode Potential Gold All other species are aqueous. Reduction reactions in acidic solution are written using h +. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. The iupac gold book defines it. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any. Standard Electrode Potential Gold.
From cbsencertsolutiononline.blogspot.com
CBSE NCERT SOLUTIONS The standard electrode potentials at 298 K Standard Electrode Potential Gold In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. 45 rows standard electrode potentials in aqueous solution at 25°c. Reduction reactions in acidic solution are written using h +. Solids, gases, and liquids are identified; All other species are aqueous. 372 rows the data below tabulates standard electrode potentials. Standard Electrode Potential Gold.
From protonstalk.com
Electrochemical Series Features, Applications, Examples ProtonsTalk Standard Electrode Potential Gold Solids, gases, and liquids are identified; Reduction reactions in acidic solution are written using h +. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The iupac gold book defines it. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most. Standard Electrode Potential Gold.
From www.pinterest.co.kr
pe=log[e] measuved in volts.if pe is negative reduced, if pe is Standard Electrode Potential Gold Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. Solids, gases, and liquids are identified; All other species are aqueous. Reduction reactions in acidic solution are written using h +. The standard cell potential is a measure of the driving force for a given redox reaction. 45. Standard Electrode Potential Gold.
From app.pandai.org
Standard Electrode Potential Standard Electrode Potential Gold Solids, gases, and liquids are identified; Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. All other species are aqueous. 45 rows standard electrode potentials in aqueous. Standard Electrode Potential Gold.
From exowfmfux.blob.core.windows.net
Standard Electrode Examples at Lucille Fitzsimmons blog Standard Electrode Potential Gold 45 rows standard electrode potentials in aqueous solution at 25°c. The iupac gold book defines it. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. Reduction reactions. Standard Electrode Potential Gold.
From unacademy.com
Electrochemical Series, Features and Importance Unacademy Standard Electrode Potential Gold 45 rows standard electrode potentials in aqueous solution at 25°c. The iupac gold book defines it. The standard cell potential is a measure of the driving force for a given redox reaction. Reduction reactions in acidic solution are written using h +. All other species are aqueous. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative. Standard Electrode Potential Gold.
From exovxcvqo.blob.core.windows.net
Standard Reduction Potential Gold at Juan Delk blog Standard Electrode Potential Gold 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The iupac gold book defines it. Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. The standard cell potential is a measure of the driving force for. Standard Electrode Potential Gold.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Electrode Potential Gold 45 rows standard electrode potentials in aqueous solution at 25°c. The iupac gold book defines it. Solids, gases, and liquids are identified; Reduction reactions in acidic solution are written using h +. The standard cell potential is a measure of the driving force for a given redox reaction. 372 rows the data below tabulates standard electrode potentials (e°), in volts. Standard Electrode Potential Gold.
From chembook.org
Standard Potential Standard Electrode Potential Gold Gold possesses very weak chemisorbing properties and an extensive double layer region that in the presence of most pure electrolytes is often. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. All other species are aqueous. The standard cell potential is a measure of the driving force for a. Standard Electrode Potential Gold.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Gold Solids, gases, and liquids are identified; Reduction reactions in acidic solution are written using h +. All other species are aqueous. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The standard cell potential is a measure of the driving force for a given redox reaction. Gold possesses very. Standard Electrode Potential Gold.