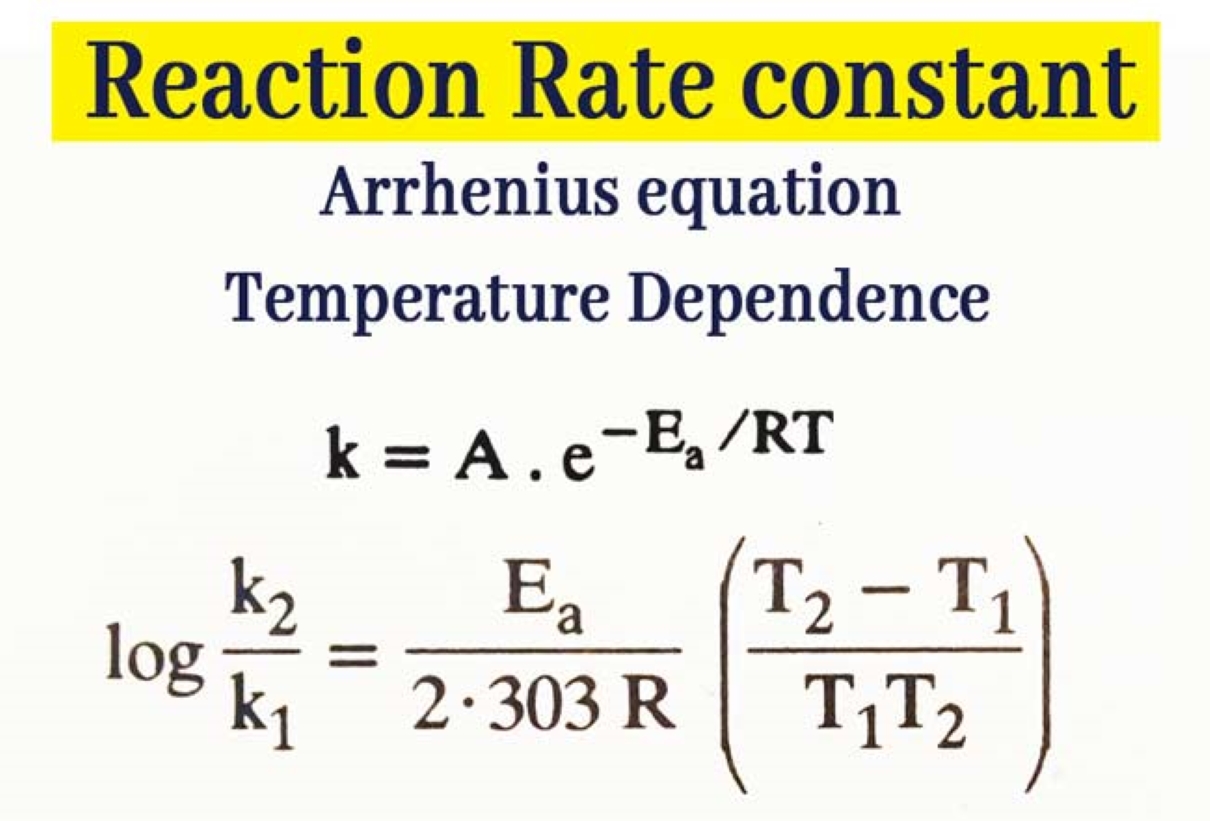
Is Rate Constant The Same As Equilibrium Constant . therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Let the equation of a reaction be as follows. as i understand, the equilibrium is actually derived from the rate constants of a reaction. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Definition of the rate constant and equilibrium constant. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows.
from facts.net
therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. as i understand, the equilibrium is actually derived from the rate constants of a reaction. Definition of the rate constant and equilibrium constant. Let the equation of a reaction be as follows. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows.
13 Mindblowing Facts About Reaction Rate Constant
Is Rate Constant The Same As Equilibrium Constant while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. Definition of the rate constant and equilibrium constant. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. as i understand, the equilibrium is actually derived from the rate constants of a reaction. Let the equation of a reaction be as follows.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Is Rate Constant The Same As Equilibrium Constant the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. Let the equation of a reaction be as follows. the equilibrium constant is equal to the rate. Is Rate Constant The Same As Equilibrium Constant.
From general.chemistrysteps.com
Equilibrium Constant Chemistry Steps Is Rate Constant The Same As Equilibrium Constant therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. while the equilibrium. Is Rate Constant The Same As Equilibrium Constant.
From askfilo.com
b) Explain the difference between equilibrium constant and rate constant... Is Rate Constant The Same As Equilibrium Constant therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Let the equation of. Is Rate Constant The Same As Equilibrium Constant.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2224637 Is Rate Constant The Same As Equilibrium Constant as i understand, the equilibrium is actually derived from the rate constants of a reaction. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Definition of the rate constant and equilibrium constant. therefore, such forward/backward reaction rates, involving initial reactants and final products, do. Is Rate Constant The Same As Equilibrium Constant.
From www.slideserve.com
PPT Chemical equilibrium occurs when a reaction and its reverse Is Rate Constant The Same As Equilibrium Constant therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. as i understand, the equilibrium is actually derived from the rate constants of a reaction. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. Definition of the rate constant and equilibrium constant. Let the equation. Is Rate Constant The Same As Equilibrium Constant.
From ecampusontario.pressbooks.pub
Chemical Equilibrium First Year General Chemistry Is Rate Constant The Same As Equilibrium Constant Let the equation of a reaction be as follows. Definition of the rate constant and equilibrium constant. as i understand, the equilibrium is actually derived from the rate constants of a reaction. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. the equilibrium constant is equal to the. Is Rate Constant The Same As Equilibrium Constant.
From www.masterorganicchemistry.com
Equilibrium and Energy Relationships Master Organic Chemistry Is Rate Constant The Same As Equilibrium Constant Let the equation of a reaction be as follows. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. the equilibrium constant is equal to the rate. Is Rate Constant The Same As Equilibrium Constant.
From slideplayer.com
Chapter 15 Chemical Equilibrium ppt download Is Rate Constant The Same As Equilibrium Constant therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. as i understand,. Is Rate Constant The Same As Equilibrium Constant.
From facts.net
13 Mindblowing Facts About Reaction Rate Constant Is Rate Constant The Same As Equilibrium Constant Let the equation of a reaction be as follows. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. Definition of the rate constant and equilibrium constant. . Is Rate Constant The Same As Equilibrium Constant.
From www.youtube.com
14.6 Chemical Equilibrium and Rate Constants YouTube Is Rate Constant The Same As Equilibrium Constant as i understand, the equilibrium is actually derived from the rate constants of a reaction. Definition of the rate constant and equilibrium constant. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by. Is Rate Constant The Same As Equilibrium Constant.
From www.slideserve.com
PPT Chapter 12 Chemical Equilibrium PowerPoint Presentation, free Is Rate Constant The Same As Equilibrium Constant at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Definition of the rate. Is Rate Constant The Same As Equilibrium Constant.
From www.researchgate.net
Reactions and equilibrium constant equations. Download Scientific Diagram Is Rate Constant The Same As Equilibrium Constant Let the equation of a reaction be as follows. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. the equilibrium constant is equal to the rate. Is Rate Constant The Same As Equilibrium Constant.
From www.youtube.com
The equilibrium constant Kp YouTube Is Rate Constant The Same As Equilibrium Constant the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Let the equation of a reaction be as follows. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. while the equilibrium constant provides information about the. Is Rate Constant The Same As Equilibrium Constant.
From secondaryscience4all.wordpress.com
1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2 Is Rate Constant The Same As Equilibrium Constant as i understand, the equilibrium is actually derived from the rate constants of a reaction. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. Let the. Is Rate Constant The Same As Equilibrium Constant.
From www.youtube.com
5.4 rate constants vs equilibrium constants YouTube Is Rate Constant The Same As Equilibrium Constant the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. while the equilibrium constant provides information about the position of the equilibrium and. Is Rate Constant The Same As Equilibrium Constant.
From learningvancetb.z14.web.core.windows.net
Chemical Equilibrium Formula Sheet Is Rate Constant The Same As Equilibrium Constant while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. Definition of the rate constant and equilibrium constant. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. the equilibrium constant is equal to the rate constant. Is Rate Constant The Same As Equilibrium Constant.
From facts.net
14 Fascinating Facts About Rate Constant Is Rate Constant The Same As Equilibrium Constant while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. at equilibrium, the. Is Rate Constant The Same As Equilibrium Constant.
From slideplayer.com
SCH4U Grade 12 Chemistry, University Preparation ppt download Is Rate Constant The Same As Equilibrium Constant Let the equation of a reaction be as follows. therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. at equilibrium, the rate of the forward and reverse reaction are equal, which. Is Rate Constant The Same As Equilibrium Constant.
From www.youtube.com
Using equilibrium constants in terms of concentrations and partial Is Rate Constant The Same As Equilibrium Constant at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. as i understand, the equilibrium is actually derived from the rate constants of a reaction. Let the equation of a reaction. Is Rate Constant The Same As Equilibrium Constant.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free Is Rate Constant The Same As Equilibrium Constant the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the. Is Rate Constant The Same As Equilibrium Constant.
From www.slideserve.com
PPT Chemistry The Science in Context Chapter 15 Chemical Equilibrium Is Rate Constant The Same As Equilibrium Constant the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction.. Is Rate Constant The Same As Equilibrium Constant.
From answerdbjack.z13.web.core.windows.net
How To Determine The Equilibrium Constant Is Rate Constant The Same As Equilibrium Constant Let the equation of a reaction be as follows. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. Definition of the rate constant and equilibrium constant. while the equilibrium constant provides information about the position. Is Rate Constant The Same As Equilibrium Constant.
From www.slideserve.com
PPT Chemical equilibrium occurs when a reaction and its reverse Is Rate Constant The Same As Equilibrium Constant at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. as i understand, the equilibrium is actually derived from the rate constants of a reaction. therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. Let the equation of a reaction be as follows. while. Is Rate Constant The Same As Equilibrium Constant.
From www.coursehero.com
[Solved] 1. Write the equilibrium constant expressions for the Is Rate Constant The Same As Equilibrium Constant therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. Let the equation of a reaction be as. Is Rate Constant The Same As Equilibrium Constant.
From www.differencebetween.com
Difference Between Equilibrium Constant and Rate Constant Compare the Is Rate Constant The Same As Equilibrium Constant Definition of the rate constant and equilibrium constant. as i understand, the equilibrium is actually derived from the rate constants of a reaction. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. the equilibrium constant is equal to the rate constant for the forward reaction divided by the. Is Rate Constant The Same As Equilibrium Constant.
From www.youtube.com
Relationship Between the Equilibrium Constants YouTube Is Rate Constant The Same As Equilibrium Constant therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. Let the equation of a reaction be as follows. as i understand, the equilibrium is actually derived from the rate constants of a reaction. the. Is Rate Constant The Same As Equilibrium Constant.
From www.masterorganicchemistry.com
Equilibrium and Energy Relationships Master Organic Chemistry Is Rate Constant The Same As Equilibrium Constant at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. as i understand,. Is Rate Constant The Same As Equilibrium Constant.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Is Rate Constant The Same As Equilibrium Constant at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. Definition of the rate constant and equilibrium constant. the equilibrium constant is equal to the rate constant for the forward reaction. Is Rate Constant The Same As Equilibrium Constant.
From www.slideserve.com
PPT Reaction Equilibrium in Ideal Gas Mixture PowerPoint Presentation Is Rate Constant The Same As Equilibrium Constant at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. as i understand, the equilibrium is actually. Is Rate Constant The Same As Equilibrium Constant.
From slideplayer.com
Reaction Rates and Equilibrium ppt download Is Rate Constant The Same As Equilibrium Constant Definition of the rate constant and equilibrium constant. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. as i understand, the equilibrium is actually derived from the rate constants of a reaction. at equilibrium, the rate of the forward and reverse reaction are equal,. Is Rate Constant The Same As Equilibrium Constant.
From www.youtube.com
Equilibrium and Reaction Rates 5 The Equilibrium Constant, Keq YouTube Is Rate Constant The Same As Equilibrium Constant while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. Let the equation of a reaction be as follows. the equilibrium constant is equal to the rate constant for the forward. Is Rate Constant The Same As Equilibrium Constant.
From classnotes.org.in
Characteristics Of Equilibrium Constant Chemistry, Class 11, Equilibrium Is Rate Constant The Same As Equilibrium Constant therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. as i understand, the equilibrium is actually derived from the rate constants of a reaction. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. the equilibrium constant is equal to. Is Rate Constant The Same As Equilibrium Constant.
From slideplayer.com
Chapter 15 Chemical Equilibrium ppt download Is Rate Constant The Same As Equilibrium Constant as i understand, the equilibrium is actually derived from the rate constants of a reaction. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. Let the equation of a reaction be as follows. Definition of the rate constant and equilibrium constant. therefore, such forward/backward reaction rates, involving initial. Is Rate Constant The Same As Equilibrium Constant.
From www.slideserve.com
PPT Chemical equilibrium occurs when a reaction and its reverse Is Rate Constant The Same As Equilibrium Constant the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Definition of the rate constant and equilibrium constant. at equilibrium, the rate of the forward and reverse reaction are equal, which is demonstrated by the arrows. the equilibrium constant is equal to the rate constant. Is Rate Constant The Same As Equilibrium Constant.
From www.slideserve.com
PPT Chemical equilibrium occurs when a reaction and its reverse Is Rate Constant The Same As Equilibrium Constant therefore, such forward/backward reaction rates, involving initial reactants and final products, do not. Let the equation of a reaction be as follows. while the equilibrium constant provides information about the position of the equilibrium and the relative stability of the. Definition of the rate constant and equilibrium constant. at equilibrium, the rate of the forward and reverse. Is Rate Constant The Same As Equilibrium Constant.