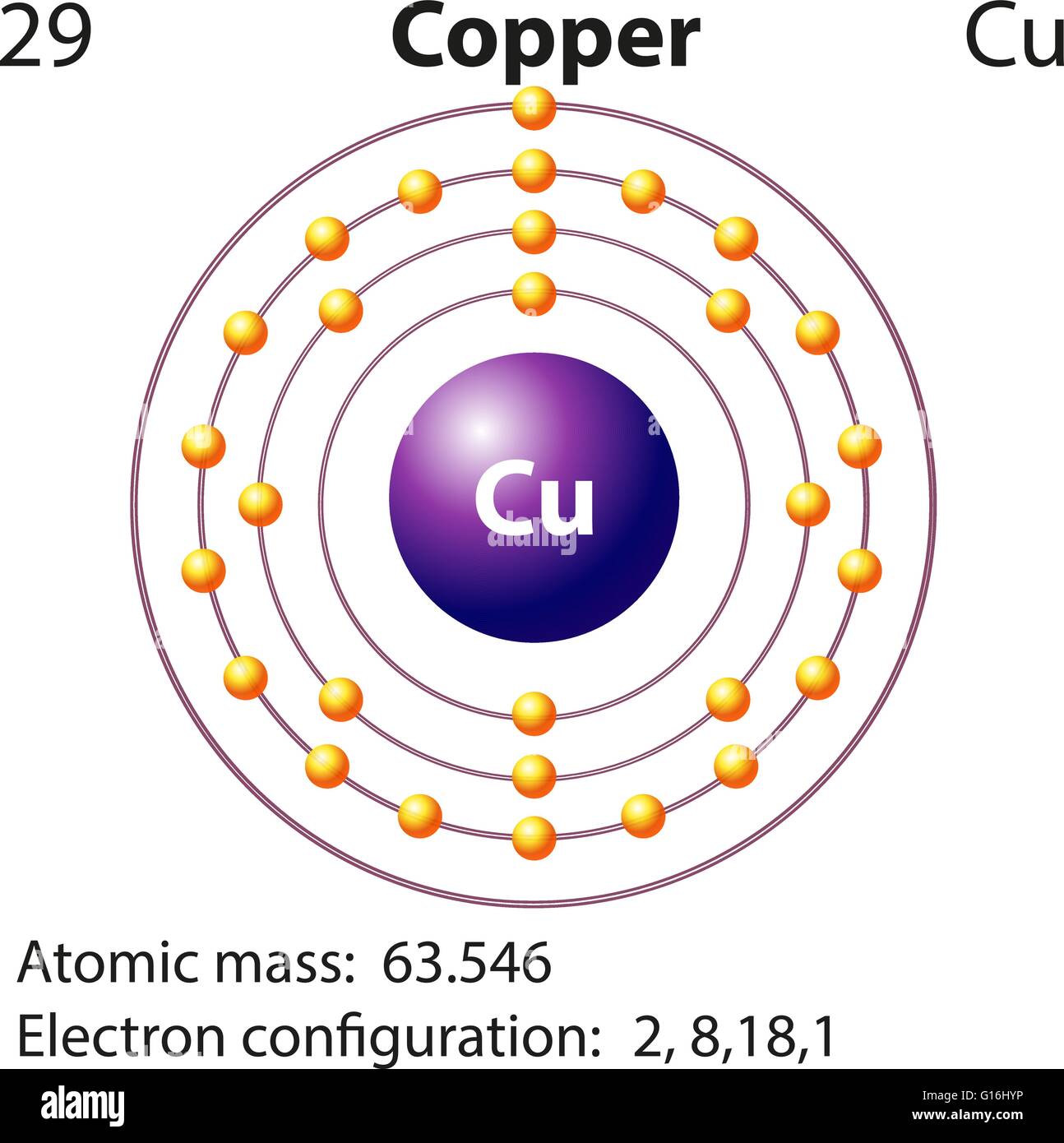
Cu Electron Configuration Class 11 . Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s }^{ 1 }{ 3d }^{ 10 }$. Learn the correct electronic configuration of copper (cu) with an explanation and a solution. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Plus, it helps to understand how covalent or ionic bonds. Each given ion contains 10 electrons. Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. This is because the 3d subshell is full, which is more stable than 3d9. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶. Find out why copper has a stable 3d10 configuration and how to. The common configuration is that of. As we see here, the. The expected configuration of cu is 4s 2 3d 9. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. Write the common electronic configuration.
from www.alamy.com
Learn the correct electronic configuration of copper (cu) with an explanation and a solution. Each given ion contains 10 electrons. Plus, it helps to understand how covalent or ionic bonds. Find out why copper has a stable 3d10 configuration and how to. The expected configuration of cu is 4s 2 3d 9. Write the common electronic configuration. As we see here, the. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s }^{ 1 }{ 3d }^{ 10 }$. Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart.
Symbol and electron diagram for Copper illustration Stock Vector Image
Cu Electron Configuration Class 11 As we see here, the. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. As we see here, the. This is because the 3d subshell is full, which is more stable than 3d9. Find out why copper has a stable 3d10 configuration and how to. Write the common electronic configuration. Plus, it helps to understand how covalent or ionic bonds. Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s }^{ 1 }{ 3d }^{ 10 }$. The common configuration is that of. Learn the correct electronic configuration of copper (cu) with an explanation and a solution. The expected configuration of cu is 4s 2 3d 9. Each given ion contains 10 electrons. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0.
From www.youtube.com
CLASS 11 CHEMISTRY ELECTRONIC CONFIGURATION YouTube Cu Electron Configuration Class 11 This is because the 3d subshell is full, which is more stable than 3d9. The common configuration is that of. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s }^{ 1 }{ 3d }^{ 10 }$. Electronic configuration refers. Cu Electron Configuration Class 11.
From www.youtube.com
Electronic Configuration class 11 chemistry chapter 2 YouTube Cu Electron Configuration Class 11 The common configuration is that of. As we see here, the. Find out why copper has a stable 3d10 configuration and how to. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. Plus, it helps to understand how covalent or ionic bonds. Write the common electronic configuration. The electronic configuration of copper. Cu Electron Configuration Class 11.
From www.youtube.com
Electron Configuration Exceptions Examples Cr, Cu, Ag, and Mo YouTube Cu Electron Configuration Class 11 As we see here, the. Find out why copper has a stable 3d10 configuration and how to. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. The common configuration is that of. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. Hence, the correct electronic configuration of cu is ${ 1s }^{. Cu Electron Configuration Class 11.
From www.youtube.com
Structure of Atom Class 11 Chemistry Discovery of Electron YouTube Cu Electron Configuration Class 11 Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶. Plus, it helps to understand how covalent or ionic bonds. Each given ion contains 10 electrons. As we see here, the. Electronic configuration. Cu Electron Configuration Class 11.
From chemistry291.blogspot.com
What Is the Electron Configuration with Step by Step Guides to write Cu Electron Configuration Class 11 The expected configuration of cu is 4s 2 3d 9. Write the common electronic configuration. Plus, it helps to understand how covalent or ionic bonds. Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s. Cu Electron Configuration Class 11.
From askfilo.com
What is the electron configuration of Cu ? Filo Cu Electron Configuration Class 11 Learn the correct electronic configuration of copper (cu) with an explanation and a solution. Find out why copper has a stable 3d10 configuration and how to. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s }^{ 1 }{ 3d. Cu Electron Configuration Class 11.
From www.youtube.com
SHORT CUT HOW TO WRITE ELECTRONIC CONFIGURATION CLASS11 Cu Electron Configuration Class 11 Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s }^{ 1 }{ 3d }^{ 10 }$. Each given ion contains 10 electrons. As we see here, the. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. The common configuration. Cu Electron Configuration Class 11.
From www.youtube.com
3 Electronic Configuration class 11 Types of Elements in periodic Cu Electron Configuration Class 11 The common configuration is that of. Plus, it helps to understand how covalent or ionic bonds. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s. Cu Electron Configuration Class 11.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes Cu Electron Configuration Class 11 Write the common electronic configuration. Each given ion contains 10 electrons. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{. Cu Electron Configuration Class 11.
From mavink.com
Electron Configuration Chart With Orbitals Cu Electron Configuration Class 11 Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Plus, it helps to understand how covalent or ionic bonds. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶. Each given ion contains. Cu Electron Configuration Class 11.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Cu Electron Configuration Class 11 Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Learn the correct electronic configuration of copper (cu) with an explanation and a solution. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2. Cu Electron Configuration Class 11.
From www.toppr.com
Write the electronic configuration of Cu(At No = 29),Cr(at no 24) and O^2 Cu Electron Configuration Class 11 This is because the 3d subshell is full, which is more stable than 3d9. Each given ion contains 10 electrons. Learn the correct electronic configuration of copper (cu) with an explanation and a solution. As we see here, the. The common configuration is that of. Find out why copper has a stable 3d10 configuration and how to. Hence, the correct. Cu Electron Configuration Class 11.
From classnotes.org.in
Electronic configuration Of Elements Chemistry, Class 11 Cu Electron Configuration Class 11 Write the common electronic configuration. As we see here, the. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s }^{ 1 }{ 3d }^{ 10 }$. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Find out why copper. Cu Electron Configuration Class 11.
From www.youtube.com
Electronic configuration.class11, classification of elements and Cu Electron Configuration Class 11 The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. As we see here, the. Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. Each given ion contains 10 electrons. The expected configuration of cu is 4s 2 3d 9. The common configuration is that of. This is because. Cu Electron Configuration Class 11.
From www.youtube.com
Electronic Configuration Electronic Configuration Class 11 Aufbau Cu Electron Configuration Class 11 Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶. Each given ion contains 10 electrons. Learn the correct electronic configuration of copper (cu) with an explanation and a solution. This is because. Cu Electron Configuration Class 11.
From www.youtube.com
Electronic configuration trick class 11 chemistry NCERT YouTube Cu Electron Configuration Class 11 Plus, it helps to understand how covalent or ionic bonds. This is because the 3d subshell is full, which is more stable than 3d9. Learn the correct electronic configuration of copper (cu) with an explanation and a solution. Find out why copper has a stable 3d10 configuration and how to. As we see here, the. Write the common electronic configuration.. Cu Electron Configuration Class 11.
From www.youtube.com
ELECTRONIC CONFIGURATION AUFBAU PRINCIPLE CLASS 11 YouTube Cu Electron Configuration Class 11 Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. Learn the correct electronic configuration of copper (cu) with an explanation and a solution. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0.. Cu Electron Configuration Class 11.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry Cu Electron Configuration Class 11 This is because the 3d subshell is full, which is more stable than 3d9. As we see here, the. Write the common electronic configuration. Plus, it helps to understand how covalent or ionic bonds. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶. Hence, the correct electronic configuration of cu is ${ 1s. Cu Electron Configuration Class 11.
From periodictable.me
Copper Electron Configuration (Cu) with Orbital Diagram Cu Electron Configuration Class 11 The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. This is because the 3d subshell is full, which is more stable than 3d9. As we see here, the. The electronic configuration of copper (cu), with an atomic number of. Cu Electron Configuration Class 11.
From www.youtube.com
electronic configuration electronic configuration class 11 Cu Electron Configuration Class 11 As we see here, the. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. Write the common electronic configuration. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. This is because the 3d subshell is full, which is more stable than 3d9. Find out why copper has a stable 3d10 configuration and. Cu Electron Configuration Class 11.
From www.youtube.com
Class11 Electronic configuration of ions ( आयनों का इलेक्ट्रानिक Cu Electron Configuration Class 11 Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s }^{ 1 }{ 3d }^{ 10 }$. Plus, it helps to understand how covalent or ionic bonds. As we see here, the. The common configuration is that of. Find out. Cu Electron Configuration Class 11.
From brainly.in
Write electronic configuration of chromium and copper. Explain why they Cu Electron Configuration Class 11 The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. Write the common electronic configuration. Plus, it helps to understand how covalent or ionic bonds. Learn the correct electronic configuration of copper (cu) with an explanation and a solution. Electronic. Cu Electron Configuration Class 11.
From www.youtube.com
Electronic Configuration of Chromium and Copper Structure of Atom Cu Electron Configuration Class 11 Electronic configuration refers to a way in order to express how electrons get arranged in an atom. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Find out why copper has a stable 3d10 configuration and how to. Each given ion contains 10 electrons. This is because the 3d subshell is full, which is more stable than 3d9. The expected. Cu Electron Configuration Class 11.
From www.alamy.com
Symbol and electron diagram for Copper illustration Stock Vector Image Cu Electron Configuration Class 11 Write the common electronic configuration. Learn the correct electronic configuration of copper (cu) with an explanation and a solution. As we see here, the. Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. Electronic configuration refers to a way in order to express how electrons get arranged in. Cu Electron Configuration Class 11.
From organicful44.blogspot.com
copper orbital diagram Organicful Cu Electron Configuration Class 11 Write the common electronic configuration. As we see here, the. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s }^{ 1 }{ 3d }^{ 10 }$. The common configuration is that of. Plus, it helps to understand how covalent. Cu Electron Configuration Class 11.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Copper (Cu) YouTube Cu Electron Configuration Class 11 Write the common electronic configuration. Each given ion contains 10 electrons. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. The common configuration is that of. Plus, it helps to understand how covalent or ionic bonds. The expected configuration of cu is 4s 2 3d 9. Learn the correct electronic configuration of. Cu Electron Configuration Class 11.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Cu Electron Configuration Class 11 Each given ion contains 10 electrons. The expected configuration of cu is 4s 2 3d 9. Write the common electronic configuration. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s. Cu Electron Configuration Class 11.
From www.youtube.com
ATOMIC STRUCTURE 05 Quantum Mechanical Model ,Quantum Numbers Cu Electron Configuration Class 11 Learn the correct electronic configuration of copper (cu) with an explanation and a solution. Plus, it helps to understand how covalent or ionic bonds. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s }^{ 1 }{ 3d }^{ 10. Cu Electron Configuration Class 11.
From www.dreamstime.com
Electron of the Element Copper Stock Vector Illustration of Cu Electron Configuration Class 11 Each given ion contains 10 electrons. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. Write the common electronic configuration. The common configuration is that of. Plus, it helps to understand how covalent or ionic bonds. As we see here, the. Learn how to write the electron configuration for copper and its. Cu Electron Configuration Class 11.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Cu Electron Configuration Class 11 Find out why copper has a stable 3d10 configuration and how to. This is because the 3d subshell is full, which is more stable than 3d9. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p. Cu Electron Configuration Class 11.
From larry-has-thornton.blogspot.com
Electronic Configuration of Elements LarryhasThornton Cu Electron Configuration Class 11 Plus, it helps to understand how covalent or ionic bonds. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. As we see here, the. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Find out why copper has a stable 3d10 configuration and how to. This is because the 3d subshell is. Cu Electron Configuration Class 11.
From classnotes.org.in
Electronic Configuration Of Elements Chemistry, Class 11, Structure Cu Electron Configuration Class 11 As we see here, the. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. This is because the 3d subshell is full, which is more stable than 3d9. Each given ion contains 10 electrons. Learn the correct electronic configuration of copper (cu) with an explanation and a solution. Plus, it helps to. Cu Electron Configuration Class 11.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Cu Electron Configuration Class 11 The common configuration is that of. Write the common electronic configuration. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶. Learn how to write the electron configuration for copper and its ions using the periodic table or an electron configuration chart. Find out why copper has a stable 3d10 configuration and how to.. Cu Electron Configuration Class 11.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Cu Electron Configuration Class 11 This is because the 3d subshell is full, which is more stable than 3d9. Electronic configuration refers to a way in order to express how electrons get arranged in an atom. The correct electronic configuration of cu+ ion is 1s22s2p63s23p63d104s14p0. Find out why copper has a stable 3d10 configuration and how to. Learn how to write the electron configuration for. Cu Electron Configuration Class 11.
From topblogtenz.com
How to write Electron Configuration All methods + Examples Cu Electron Configuration Class 11 Plus, it helps to understand how covalent or ionic bonds. The electronic configuration of copper (cu), with an atomic number of 29, is 1s² 2s² 2p⁶. Hence, the correct electronic configuration of cu is ${ 1s }^{ 2 }{ 2s }^{ 2 }{ 2p }^{ 6 }{ 3s }^{ 2 }{ 3p }^{ 6 }{ 4s }^{ 1 }{ 3d. Cu Electron Configuration Class 11.