Equation For Dilutions . — write the dilution equation. C 1 v 1 = c 2 v 2 where: As a result, this gives us a way to. Apply the dilution equation to calculate the final concentration, or the. — learn how to dilute and concentrate solutions. explain how concentrations can be changed in the lab. this yields the most general form of the dilution equation: \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. Understand how stock solutions are used in the laboratory. The property that the amount of solute does not change on dilution and concentration gives us a way. — to make a fixed amount of a dilute solution from a stock solution, you can use the formula: the formula for dilution: — dilution formula. Often, a worker will need to change the concentration of a. In both the dilution and concentration processes, the amount of solute stays the same.
from www.slideshare.net
As a result, this gives us a way to. C 1 v 1 = c 2 v 2 where: — dilution formula. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. Understand how stock solutions are used in the laboratory. explain how concentrations can be changed in the lab. — learn how to dilute and concentrate solutions. this yields the most general form of the dilution equation: The property that the amount of solute does not change on dilution and concentration gives us a way. — to make a fixed amount of a dilute solution from a stock solution, you can use the formula:
Molarity and dilution
Equation For Dilutions the formula for dilution: Apply the dilution equation to calculate the final concentration, or the. The property that the amount of solute does not change on dilution and concentration gives us a way. — to make a fixed amount of a dilute solution from a stock solution, you can use the formula: \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. In both the dilution and concentration processes, the amount of solute stays the same. Understand how stock solutions are used in the laboratory. C 1 v 1 = c 2 v 2 where: explain how concentrations can be changed in the lab. this yields the most general form of the dilution equation: — dilution formula. Often, a worker will need to change the concentration of a. the formula for dilution: — write the dilution equation. — learn how to dilute and concentrate solutions. As a result, this gives us a way to.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Equation For Dilutions Often, a worker will need to change the concentration of a. The property that the amount of solute does not change on dilution and concentration gives us a way. the formula for dilution: — dilution formula. explain how concentrations can be changed in the lab. — to make a fixed amount of a dilute solution from. Equation For Dilutions.
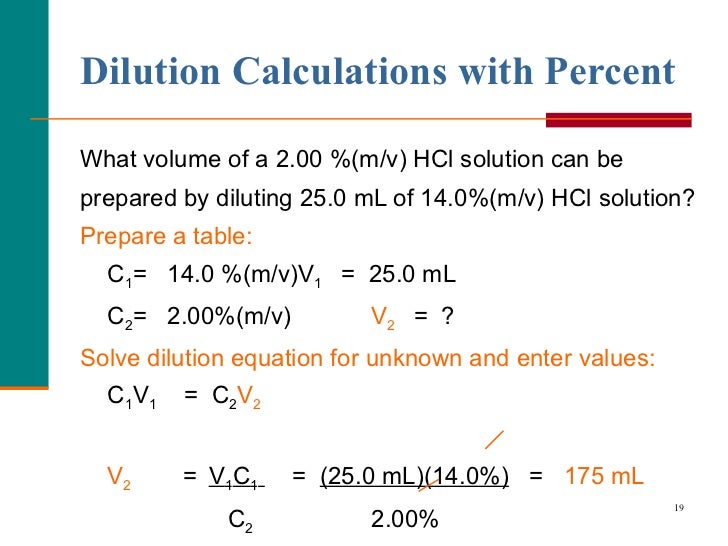
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Equation For Dilutions the formula for dilution: As a result, this gives us a way to. In both the dilution and concentration processes, the amount of solute stays the same. — learn how to dilute and concentrate solutions. — to make a fixed amount of a dilute solution from a stock solution, you can use the formula: Apply the dilution. Equation For Dilutions.
From www.youtube.com
DILUTION AND MIXING OF SOLUTIONS, MOLARITY EQUATION /SOME BASIC Equation For Dilutions C 1 v 1 = c 2 v 2 where: The property that the amount of solute does not change on dilution and concentration gives us a way. explain how concentrations can be changed in the lab. — write the dilution equation. Understand how stock solutions are used in the laboratory. As a result, this gives us a. Equation For Dilutions.
From learningisidro.z13.web.core.windows.net
Making Dilutions Worksheets Equation For Dilutions — dilution formula. In both the dilution and concentration processes, the amount of solute stays the same. The property that the amount of solute does not change on dilution and concentration gives us a way. this yields the most general form of the dilution equation: C 1 v 1 = c 2 v 2 where: — learn. Equation For Dilutions.
From www.pdfprof.com
how to calculate dilution factor Equation For Dilutions In both the dilution and concentration processes, the amount of solute stays the same. the formula for dilution: this yields the most general form of the dilution equation: — write the dilution equation. — dilution formula. explain how concentrations can be changed in the lab. The property that the amount of solute does not change. Equation For Dilutions.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Equation For Dilutions — to make a fixed amount of a dilute solution from a stock solution, you can use the formula: — write the dilution equation. — learn how to dilute and concentrate solutions. Apply the dilution equation to calculate the final concentration, or the. As a result, this gives us a way to. this yields the most. Equation For Dilutions.
From www.youtube.com
How to Calculate Dilution Factor YouTube Equation For Dilutions the formula for dilution: explain how concentrations can be changed in the lab. this yields the most general form of the dilution equation: — learn how to dilute and concentrate solutions. — dilution formula. — write the dilution equation. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume.. Equation For Dilutions.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Equation For Dilutions In both the dilution and concentration processes, the amount of solute stays the same. the formula for dilution: explain how concentrations can be changed in the lab. — write the dilution equation. Often, a worker will need to change the concentration of a. Understand how stock solutions are used in the laboratory. — to make a. Equation For Dilutions.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Equation For Dilutions — learn how to dilute and concentrate solutions. explain how concentrations can be changed in the lab. — dilution formula. this yields the most general form of the dilution equation: — write the dilution equation. Apply the dilution equation to calculate the final concentration, or the. — to make a fixed amount of a. Equation For Dilutions.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Equation For Dilutions As a result, this gives us a way to. Understand how stock solutions are used in the laboratory. Apply the dilution equation to calculate the final concentration, or the. the formula for dilution: C 1 v 1 = c 2 v 2 where: — to make a fixed amount of a dilute solution from a stock solution, you. Equation For Dilutions.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Equation For Dilutions The property that the amount of solute does not change on dilution and concentration gives us a way. As a result, this gives us a way to. — dilution formula. — to make a fixed amount of a dilute solution from a stock solution, you can use the formula: the formula for dilution: C 1 v 1. Equation For Dilutions.
From www.showme.com
Dilution calculation Science, Chemicalreactions, Biology ShowMe Equation For Dilutions Often, a worker will need to change the concentration of a. As a result, this gives us a way to. — dilution formula. Understand how stock solutions are used in the laboratory. — learn how to dilute and concentrate solutions. In both the dilution and concentration processes, the amount of solute stays the same. \[c_1v_1=c_2v_2\] to summarize, c. Equation For Dilutions.
From exookhlfi.blob.core.windows.net
Dilution Factor Calculation In Hplc at John Comer blog Equation For Dilutions Apply the dilution equation to calculate the final concentration, or the. — learn how to dilute and concentrate solutions. — to make a fixed amount of a dilute solution from a stock solution, you can use the formula: — dilution formula. explain how concentrations can be changed in the lab. C 1 v 1 = c. Equation For Dilutions.
From exorkoxox.blob.core.windows.net
Dilution Volume Formula at Callie Douglass blog Equation For Dilutions \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. C 1 v 1 = c 2 v 2 where: Apply the dilution equation to calculate the final concentration, or the. As a result, this gives us a way to. this yields the most general form of the dilution equation: explain how concentrations. Equation For Dilutions.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Equation For Dilutions — dilution formula. — to make a fixed amount of a dilute solution from a stock solution, you can use the formula: this yields the most general form of the dilution equation: The property that the amount of solute does not change on dilution and concentration gives us a way. \[c_1v_1=c_2v_2\] to summarize, c stands for the. Equation For Dilutions.
From www.slideshare.net
Molarity and dilution Equation For Dilutions C 1 v 1 = c 2 v 2 where: — learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. explain how concentrations can be changed in the lab. this yields the most general form of the dilution equation: Often, a worker will need to change the concentration of a.. Equation For Dilutions.
From ar.inspiredpencil.com
Dilution Equation Equation For Dilutions explain how concentrations can be changed in the lab. As a result, this gives us a way to. — dilution formula. — to make a fixed amount of a dilute solution from a stock solution, you can use the formula: In both the dilution and concentration processes, the amount of solute stays the same. Understand how stock. Equation For Dilutions.
From www.youtube.com
Dilution in biology lab How to use the equation C1V1 = C2V2 YouTube Equation For Dilutions In both the dilution and concentration processes, the amount of solute stays the same. Understand how stock solutions are used in the laboratory. — write the dilution equation. this yields the most general form of the dilution equation: — learn how to dilute and concentrate solutions. The property that the amount of solute does not change on. Equation For Dilutions.
From www.youtube.com
Dilution Equation 1 YouTube Equation For Dilutions \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. In both the dilution and concentration processes, the amount of solute stays the same. As a result, this gives us a way to. this yields the most general form of the dilution equation: Apply the dilution equation to calculate the final concentration, or the.. Equation For Dilutions.
From www.educba.com
Dilution Formula Calculator (Examples with Excel Template) Equation For Dilutions Often, a worker will need to change the concentration of a. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. — dilution formula. Understand how stock solutions are used in the laboratory. this yields the most general form of the dilution equation: — write the dilution equation. — to make. Equation For Dilutions.
From chem2u.blogspot.com
chem2U Dilution method formula Equation For Dilutions C 1 v 1 = c 2 v 2 where: In both the dilution and concentration processes, the amount of solute stays the same. Apply the dilution equation to calculate the final concentration, or the. explain how concentrations can be changed in the lab. this yields the most general form of the dilution equation: As a result, this. Equation For Dilutions.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Equation For Dilutions — learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. — dilution formula. this yields the most general form of the dilution equation: In both the dilution and concentration processes, the amount of solute stays the same. explain how concentrations can be changed in the lab. Often, a worker. Equation For Dilutions.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Equation For Dilutions — dilution formula. In both the dilution and concentration processes, the amount of solute stays the same. — write the dilution equation. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. The property that the amount of solute does not change on dilution and concentration gives us a way. the formula. Equation For Dilutions.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Equation For Dilutions this yields the most general form of the dilution equation: As a result, this gives us a way to. In both the dilution and concentration processes, the amount of solute stays the same. The property that the amount of solute does not change on dilution and concentration gives us a way. — dilution formula. — write the. Equation For Dilutions.
From www.slideserve.com
PPT Chapter 1 The Organization of Matter PowerPoint Presentation Equation For Dilutions the formula for dilution: — dilution formula. As a result, this gives us a way to. In both the dilution and concentration processes, the amount of solute stays the same. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. — to make a fixed amount of a dilute solution from a. Equation For Dilutions.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Equation For Dilutions — write the dilution equation. explain how concentrations can be changed in the lab. C 1 v 1 = c 2 v 2 where: Often, a worker will need to change the concentration of a. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. — dilution formula. this yields the. Equation For Dilutions.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Equation For Dilutions Understand how stock solutions are used in the laboratory. As a result, this gives us a way to. C 1 v 1 = c 2 v 2 where: — write the dilution equation. In both the dilution and concentration processes, the amount of solute stays the same. this yields the most general form of the dilution equation: . Equation For Dilutions.
From dxohqxlgl.blob.core.windows.net
Dilution 1 Volume at Jeremiah Gonzalez blog Equation For Dilutions — dilution formula. Often, a worker will need to change the concentration of a. In both the dilution and concentration processes, the amount of solute stays the same. Understand how stock solutions are used in the laboratory. — to make a fixed amount of a dilute solution from a stock solution, you can use the formula: \[c_1v_1=c_2v_2\] to. Equation For Dilutions.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Equation For Dilutions Apply the dilution equation to calculate the final concentration, or the. explain how concentrations can be changed in the lab. The property that the amount of solute does not change on dilution and concentration gives us a way. Often, a worker will need to change the concentration of a. — dilution formula. Understand how stock solutions are used. Equation For Dilutions.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Equation For Dilutions — dilution formula. — to make a fixed amount of a dilute solution from a stock solution, you can use the formula: — write the dilution equation. — learn how to dilute and concentrate solutions. As a result, this gives us a way to. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v. Equation For Dilutions.
From exorkoxox.blob.core.windows.net
Dilution Volume Formula at Callie Douglass blog Equation For Dilutions — learn how to dilute and concentrate solutions. C 1 v 1 = c 2 v 2 where: this yields the most general form of the dilution equation: The property that the amount of solute does not change on dilution and concentration gives us a way. Often, a worker will need to change the concentration of a. . Equation For Dilutions.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Equation For Dilutions the formula for dilution: this yields the most general form of the dilution equation: C 1 v 1 = c 2 v 2 where: Often, a worker will need to change the concentration of a. Apply the dilution equation to calculate the final concentration, or the. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v. Equation For Dilutions.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Equation For Dilutions As a result, this gives us a way to. Often, a worker will need to change the concentration of a. The property that the amount of solute does not change on dilution and concentration gives us a way. — write the dilution equation. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. C. Equation For Dilutions.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Equation For Dilutions — learn how to dilute and concentrate solutions. The property that the amount of solute does not change on dilution and concentration gives us a way. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. explain how concentrations can be changed in the lab. — to make a fixed amount of. Equation For Dilutions.
From www.slideshare.net
Molarity and dilution Equation For Dilutions explain how concentrations can be changed in the lab. — dilution formula. this yields the most general form of the dilution equation: — write the dilution equation. — learn how to dilute and concentrate solutions. the formula for dilution: In both the dilution and concentration processes, the amount of solute stays the same. Often,. Equation For Dilutions.