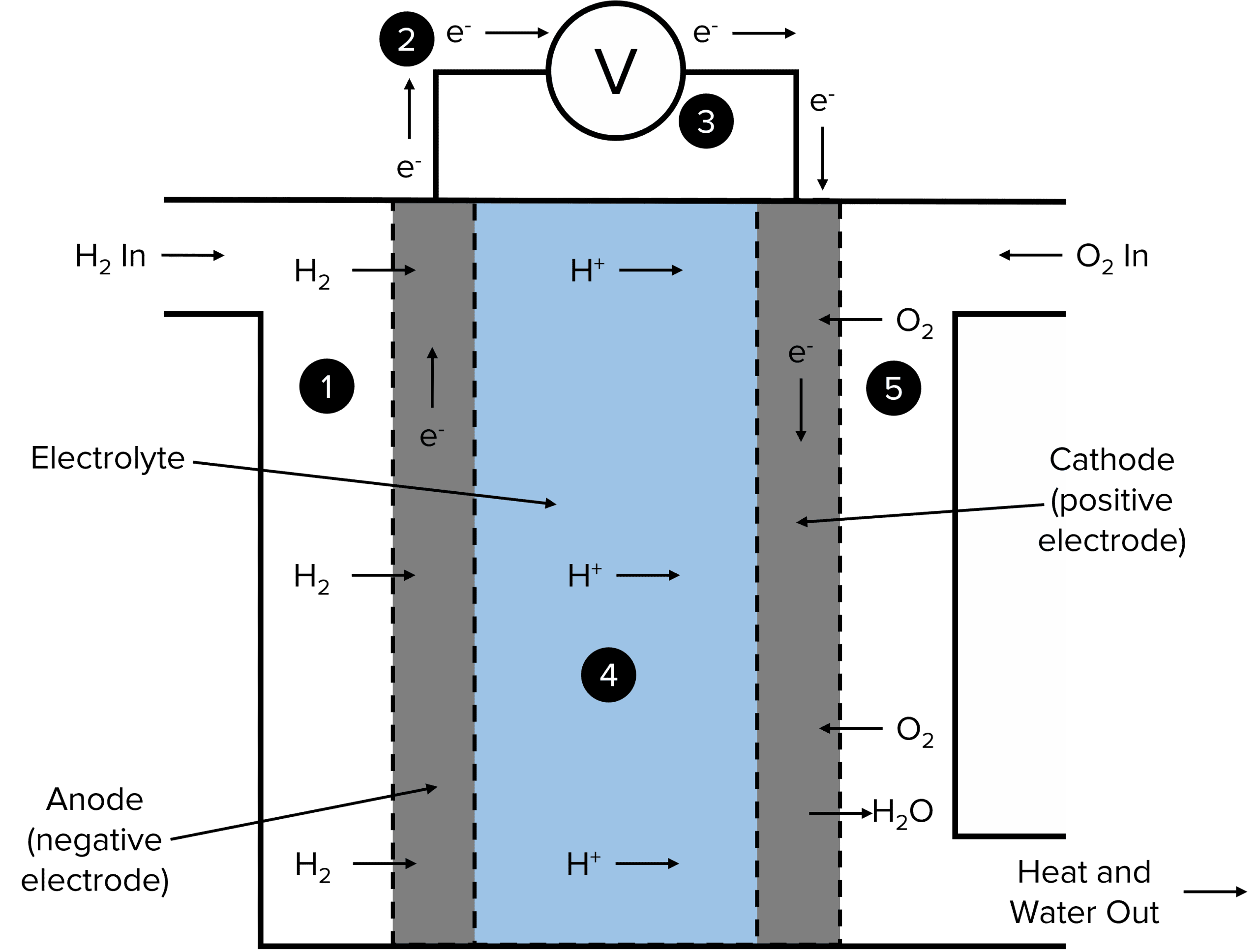
Fuel Cells In Chemistry . A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Unlike a battery, it does not store. Fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Fuel cells have an important advantage over all. Work in a different way than.
from mmerevise.co.uk
Fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage over all. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Work in a different way than. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Unlike a battery, it does not store. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed.
Electrochemical Cells Worksheets and Revision MME
Fuel Cells In Chemistry A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Work in a different way than. Fuel cells have an important advantage over all. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Unlike a battery, it does not store.
From www.britannica.com
Fuel cell Proton Exchange, Alkaline, Polymer Britannica Fuel Cells In Chemistry A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell is a galvanic cell that requires a constant external supply of. Fuel Cells In Chemistry.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cells In Chemistry Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Fuel cells have an important advantage over all. Unlike a battery, it does not store. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed.. Fuel Cells In Chemistry.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Cells In Chemistry A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Unlike a battery, it does not store. A fuel cell is a galvanic cell. Fuel Cells In Chemistry.
From iupac.org
Fuel Cells IUPAC International Union of Pure and Applied Chemistry Fuel Cells In Chemistry Unlike a battery, it does not store. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. Work in a different way than. Fuel cell, any of a. Fuel Cells In Chemistry.
From courses.lumenlearning.com
Phases and Classification of Matter Introductory Chemistry Lecture Fuel Cells In Chemistry Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly. Fuel Cells In Chemistry.
From pressbooks.openedmb.ca
Batteries and Fuel Cells Chemistry and the Environment Fuel Cells In Chemistry A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cell, any of a class of devices that convert the chemical energy of. Fuel Cells In Chemistry.
From www.savemyexams.com
Fuel Cells SL IB Chemistry Revision Notes 2025 Fuel Cells In Chemistry Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Fuel cells have an important advantage over all. Fuel cells close fuel cell device that produces a. Fuel Cells In Chemistry.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room Fuel Cells In Chemistry A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell is a galvanic cell that requires a constant external supply of. Fuel Cells In Chemistry.
From stock.adobe.com
Fuel Cell Solid Oxide Diagram Stock Vector Adobe Stock Fuel Cells In Chemistry Fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. Unlike a battery, it does not store. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell like this will continue to operate and produce electrical energy. Fuel Cells In Chemistry.
From www.researchgate.net
6. Hydrogenoxygen fuel cell scheme. Download Scientific Diagram Fuel Cells In Chemistry Unlike a battery, it does not store. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is an electrochemical cell. Fuel Cells In Chemistry.
From www.tes.com
Electrolysis and Fuel Cells GCSE Chemistry Teaching Resources Fuel Cells In Chemistry Fuel cells have an important advantage over all. Work in a different way than. Unlike a battery, it does not store. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cells are similar to batteries in that they generate an electrical current, but require. Fuel Cells In Chemistry.
From www.tes.com
Chemical Cells and Fuel Cells GCSE AQA Chemistry HIGHER Teaching Fuel Cells In Chemistry Fuel cells have an important advantage over all. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Unlike a battery, it does not store. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel. Fuel Cells In Chemistry.
From chem.libretexts.org
Case Study Fuel Cells Chemistry LibreTexts Fuel Cells In Chemistry A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells have an important advantage over all. A fuel cell is an electrochemical. Fuel Cells In Chemistry.
From chem.libretexts.org
6.7 Batteries and Fuel Cells Chemistry LibreTexts Fuel Cells In Chemistry Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Work in a different way than. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A fuel cell like this will continue to operate. Fuel Cells In Chemistry.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cells In Chemistry A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. A fuel cell is a galvanic cell that requires a constant external supply of reactants. Fuel Cells In Chemistry.
From www.youtube.com
GCSE Chemistry Fuel Cells 45 YouTube Fuel Cells In Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell like this will continue to operate and produce electrical energy as long as. Fuel Cells In Chemistry.
From www.researchgate.net
Basic structure of PEM fuel cell Download Scientific Diagram Fuel Cells In Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells close fuel cell device that produces a voltage continuously when supplied with. Fuel Cells In Chemistry.
From www.youtube.com
GCSE Chemistry Fuel Cells YouTube Fuel Cells In Chemistry Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Work in a different way than. Fuel cells have an important advantage over all. A fuel cell. Fuel Cells In Chemistry.
From courses.lumenlearning.com
Applications of Redox Reactions Voltaic Cells Introductory Chemistry Fuel Cells In Chemistry Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Fuel cells have an important advantage over all. Work in a different way than. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell. Fuel Cells In Chemistry.
From revisechemistry.uk
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk Fuel Cells In Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A fuel cell like this will continue to operate and produce electrical energy as. Fuel Cells In Chemistry.
From www.youtube.com
AQA GCSE Chemistry P1 Hydrogen Fuel Cells YouTube Fuel Cells In Chemistry A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Work in a different way than. Fuel cells have an important advantage over all. Unlike a battery, it does not store. A fuel cell resembles a battery in many respects, but it can supply electrical energy. Fuel Cells In Chemistry.
From www.youtube.com
Direct Methanol Fuel Cell, Introduction, Principle, Advantages Fuel Cells In Chemistry Fuel cells have an important advantage over all. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. A fuel cell like this will continue. Fuel Cells In Chemistry.
From www.nfcrc.uci.edu
National Fuel Cell Research Center (NFCRC), UC Irvine Fuel Cells In Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen. Fuel Cells In Chemistry.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cells In Chemistry A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Work in a different way than. Unlike a battery, it does not store. Fuel cell,. Fuel Cells In Chemistry.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cells In Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. Unlike a battery, it does not store. Fuel cells are similar to batteries in that they generate an electrical current,. Fuel Cells In Chemistry.
From chemistry.stackexchange.com
electrochemistry Electrode polarity in fuel cells Chemistry Stack Fuel Cells In Chemistry Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Work in a different way than. A fuel cell is an electrochemical cell in which a fuel. Fuel Cells In Chemistry.
From www.youtube.com
OCR C6 Fuel Cells (Higher) YouTube Fuel Cells In Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Work in a different way than. Fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. Fuel cells are similar to batteries in that they generate an electrical current, but. Fuel Cells In Chemistry.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cells In Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Unlike a battery, it does not store. Work in a different way than. A. Fuel Cells In Chemistry.
From www.linquip.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip Fuel Cells In Chemistry A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. A fuel cell resembles a battery in many respects, but it can supply electrical energy. Fuel Cells In Chemistry.
From www.vhv.rs
Fuel Cell Block Diagram Hydrogen Fuel Cells Aqa Chemistry Gcse, HD Fuel Cells In Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Unlike a battery, it does not store. Work in a different way than. Fuel cells close. Fuel Cells In Chemistry.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Fuel Cells In Chemistry A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Fuel cell, any of a class of devices that convert the chemical. Fuel Cells In Chemistry.
From www.e-conversion.de
Fuel cell principle econversion Fuel Cells In Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells are similar to batteries in that they generate an electrical current, but. Fuel Cells In Chemistry.
From studymind.co.uk
Fuel Cells (GCSE Chemistry) Study Mind Fuel Cells In Chemistry Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Fuel cells have an important advantage over all. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells close fuel cell device that produces a. Fuel Cells In Chemistry.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Fuel Cells In Chemistry Unlike a battery, it does not store. Fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. Work in a different way than. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells have an important advantage over. Fuel Cells In Chemistry.
From greenfuture.io
What Are Hydrogen Fuel Cell Cars? Fuel Cells In Chemistry A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Work in a different way than. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell is a galvanic cell that. Fuel Cells In Chemistry.