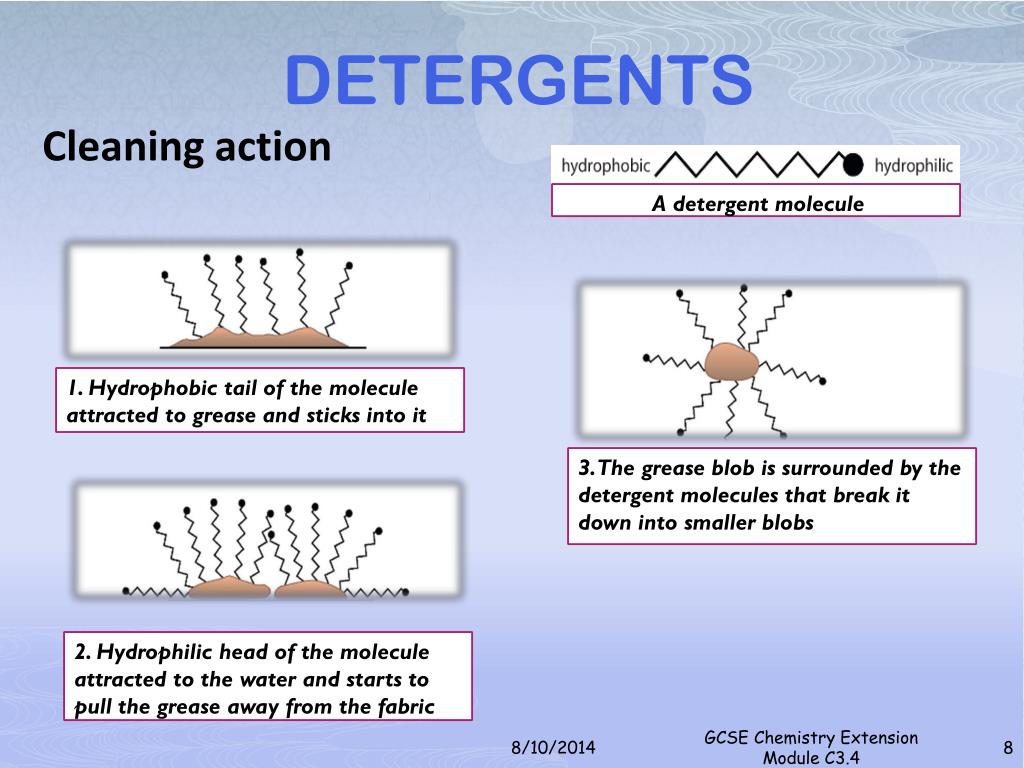
Water Surface Tension Detergents . Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more likely to interact with oil and grease. Detergent molecules have two ends, one hydrophobic end, repelled by water and one hydrophilic end, attracted to water. This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to the phenomenon of surface tension,. However, most people also rinse the dishes with pure However, the science behind how it. The reduced surface tension of dishwashing water, and increasing solubility of modern surfactant mixtures, allows the water to run off the dishes in a dish rack very quickly. Detergents lower the surface tension of water. Upon putting a drop of soap solution/detergent on the notch, boat accelerates. The end of the detergent molecule which attaches to fat (grease) repels water molecules. When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces. Detergents are primarily surfactants, which could be produced easily from petrochemicals. Modern detergents contain more than surfactants. Detergent molecules' two ends make it able to break through the surface tension of water. To make water wash better, we have to reduce its surface tension so it wets things more uniformly. And that's precisely what a surfactant.
from www.slideserve.com
Detergent molecules' two ends make it able to break through the surface tension of water. Upon putting a drop of soap solution/detergent on the notch, boat accelerates. The reduced surface tension of dishwashing water, and increasing solubility of modern surfactant mixtures, allows the water to run off the dishes in a dish rack very quickly. The end of the detergent molecule which attaches to fat (grease) repels water molecules. Detergents are primarily surfactants, which could be produced easily from petrochemicals. Soap is a commonly used cleaning agent that helps remove dirt and grime from various surfaces. Detergents lower the surface tension of water. When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces. Detergent molecules have two ends, one hydrophobic end, repelled by water and one hydrophilic end, attracted to water. This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to the phenomenon of surface tension,.
PPT C3.4.14 Detergents PowerPoint Presentation, free download ID
Water Surface Tension Detergents And that's precisely what a surfactant. Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more likely to interact with oil and grease. Detergents are primarily surfactants, which could be produced easily from petrochemicals. Detergents lower the surface tension of water. When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces. The reduced surface tension of dishwashing water, and increasing solubility of modern surfactant mixtures, allows the water to run off the dishes in a dish rack very quickly. This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to the phenomenon of surface tension,. The end of the detergent molecule which attaches to fat (grease) repels water molecules. Detergent molecules' two ends make it able to break through the surface tension of water. Modern detergents contain more than surfactants. Soap is a commonly used cleaning agent that helps remove dirt and grime from various surfaces. Upon putting a drop of soap solution/detergent on the notch, boat accelerates. However, most people also rinse the dishes with pure Detergent molecules have two ends, one hydrophobic end, repelled by water and one hydrophilic end, attracted to water. To make water wash better, we have to reduce its surface tension so it wets things more uniformly. However, the science behind how it.
From materialmcgheeferried.z21.web.core.windows.net
How To Explain Surface Tension Water Surface Tension Detergents Soap is a commonly used cleaning agent that helps remove dirt and grime from various surfaces. Detergents are primarily surfactants, which could be produced easily from petrochemicals. To make water wash better, we have to reduce its surface tension so it wets things more uniformly. Detergent molecules have two ends, one hydrophobic end, repelled by water and one hydrophilic end,. Water Surface Tension Detergents.
From www.youtube.com
Detergents and Surface Tension [ Ch10 , L18 ] YouTube Water Surface Tension Detergents However, most people also rinse the dishes with pure To make water wash better, we have to reduce its surface tension so it wets things more uniformly. Upon putting a drop of soap solution/detergent on the notch, boat accelerates. The end of the detergent molecule which attaches to fat (grease) repels water molecules. However, the science behind how it. Surfactants. Water Surface Tension Detergents.
From www.youtube.com
DETERMINING THE SURFACE TENSION OF WATER EASY TO UNDERSTAND YouTube Water Surface Tension Detergents Detergent molecules' two ends make it able to break through the surface tension of water. Upon putting a drop of soap solution/detergent on the notch, boat accelerates. The reduced surface tension of dishwashing water, and increasing solubility of modern surfactant mixtures, allows the water to run off the dishes in a dish rack very quickly. However, the science behind how. Water Surface Tension Detergents.
From www.biolinscientific.com
What is a wetting agent and where are they used? Water Surface Tension Detergents However, the science behind how it. Detergent molecules' two ends make it able to break through the surface tension of water. Detergents are primarily surfactants, which could be produced easily from petrochemicals. When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces. To make water wash better,. Water Surface Tension Detergents.
From www.youtube.com
What is Water Pollution ? Detergents Surface Tension Pont source Water Surface Tension Detergents Detergents lower the surface tension of water. This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to the phenomenon of surface tension,. And that's precisely what a surfactant. Soap is a commonly used cleaning agent that helps remove dirt and grime from various surfaces.. Water Surface Tension Detergents.
From www.youtube.com
Soap and the effect it has on the Surface Tension of Water YouTube Water Surface Tension Detergents Detergent molecules have two ends, one hydrophobic end, repelled by water and one hydrophilic end, attracted to water. This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to the phenomenon of surface tension,. Detergent molecules' two ends make it able to break through the. Water Surface Tension Detergents.
From edu.rsc.org
Detergents, soaps and surface tension Experiment RSC Education Water Surface Tension Detergents When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces. This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to the phenomenon of surface tension,. Surfactants lower the surface tension of. Water Surface Tension Detergents.
From slideplayer.com
1. ppt download Water Surface Tension Detergents To make water wash better, we have to reduce its surface tension so it wets things more uniformly. However, the science behind how it. The end of the detergent molecule which attaches to fat (grease) repels water molecules. The reduced surface tension of dishwashing water, and increasing solubility of modern surfactant mixtures, allows the water to run off the dishes. Water Surface Tension Detergents.
From thevigyan.com
Why does adding detergent to water lower its surface tension? The Vigyan Water Surface Tension Detergents When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces. Detergents are primarily surfactants, which could be produced easily from petrochemicals. This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to. Water Surface Tension Detergents.
From www.slideserve.com
PPT C3.4.14 Detergents PowerPoint Presentation, free download ID Water Surface Tension Detergents However, most people also rinse the dishes with pure Detergents are primarily surfactants, which could be produced easily from petrochemicals. Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more likely to interact with oil and grease. The reduced surface tension of dishwashing water, and increasing solubility. Water Surface Tension Detergents.
From www.wikihow.life
How to Study the Chemistry of Detergents 12 Steps (with Pictures) Water Surface Tension Detergents To make water wash better, we have to reduce its surface tension so it wets things more uniformly. The reduced surface tension of dishwashing water, and increasing solubility of modern surfactant mixtures, allows the water to run off the dishes in a dish rack very quickly. Upon putting a drop of soap solution/detergent on the notch, boat accelerates. Detergent molecules'. Water Surface Tension Detergents.
From myluvlychemistry.blogspot.com
my chemistry Cleansing Action Of Soap and Detergent Water Surface Tension Detergents Detergent molecules' two ends make it able to break through the surface tension of water. However, most people also rinse the dishes with pure This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to the phenomenon of surface tension,. And that's precisely what a. Water Surface Tension Detergents.
From www.toppr.com
Adding detergents to water helps in removing dirty greasy stains. This Water Surface Tension Detergents Detergents lower the surface tension of water. The reduced surface tension of dishwashing water, and increasing solubility of modern surfactant mixtures, allows the water to run off the dishes in a dish rack very quickly. Detergent molecules' two ends make it able to break through the surface tension of water. However, the science behind how it. Detergents are primarily surfactants,. Water Surface Tension Detergents.
From www.researchgate.net
Foam volume, surface tension at 0.1 conc. of detergent in water Water Surface Tension Detergents To make water wash better, we have to reduce its surface tension so it wets things more uniformly. Detergent molecules have two ends, one hydrophobic end, repelled by water and one hydrophilic end, attracted to water. Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more likely. Water Surface Tension Detergents.
From www.youtube.com
Detergent surface tension Part 2 Water droplets on baking paper Water Surface Tension Detergents The reduced surface tension of dishwashing water, and increasing solubility of modern surfactant mixtures, allows the water to run off the dishes in a dish rack very quickly. Detergent molecules have two ends, one hydrophobic end, repelled by water and one hydrophilic end, attracted to water. However, the science behind how it. When the boat is placed gently on the. Water Surface Tension Detergents.
From www.alamy.com
The presence in water of certain surfactant substances, such as soaps Water Surface Tension Detergents Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more likely to interact with oil and grease. When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces. Soap is a commonly used cleaning agent. Water Surface Tension Detergents.
From slideplayer.com
Chapter 10 States of Matter & Water Cycle ppt download Water Surface Tension Detergents Soap is a commonly used cleaning agent that helps remove dirt and grime from various surfaces. And that's precisely what a surfactant. Detergent molecules' two ends make it able to break through the surface tension of water. When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces.. Water Surface Tension Detergents.
From www.slideserve.com
PPT Chapter 30 Detergents PowerPoint Presentation, free download ID Water Surface Tension Detergents The reduced surface tension of dishwashing water, and increasing solubility of modern surfactant mixtures, allows the water to run off the dishes in a dish rack very quickly. The end of the detergent molecule which attaches to fat (grease) repels water molecules. To make water wash better, we have to reduce its surface tension so it wets things more uniformly.. Water Surface Tension Detergents.
From www.doubtnut.com
Detergents increase the surface tension of water Water Surface Tension Detergents To make water wash better, we have to reduce its surface tension so it wets things more uniformly. The end of the detergent molecule which attaches to fat (grease) repels water molecules. Detergents lower the surface tension of water. And that's precisely what a surfactant. This series of brief experiments on the surface tension of water, and the effects of. Water Surface Tension Detergents.
From askfilo.com
Detergents and Surface tension The addition of the substances like soap a.. Water Surface Tension Detergents To make water wash better, we have to reduce its surface tension so it wets things more uniformly. When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces. Soap is a commonly used cleaning agent that helps remove dirt and grime from various surfaces. This series of. Water Surface Tension Detergents.
From sites.udel.edu
How to Teach Your Kids Science and Engineering K12 Engineering Water Surface Tension Detergents Modern detergents contain more than surfactants. The end of the detergent molecule which attaches to fat (grease) repels water molecules. The reduced surface tension of dishwashing water, and increasing solubility of modern surfactant mixtures, allows the water to run off the dishes in a dish rack very quickly. Detergents lower the surface tension of water. Detergent molecules have two ends,. Water Surface Tension Detergents.
From kidpillar.com
What is Surface Tension + Fun Experiments on Surface Tension Water Surface Tension Detergents Detergents are primarily surfactants, which could be produced easily from petrochemicals. Detergent molecules have two ends, one hydrophobic end, repelled by water and one hydrophilic end, attracted to water. Detergents lower the surface tension of water. Upon putting a drop of soap solution/detergent on the notch, boat accelerates. However, most people also rinse the dishes with pure Surfactants lower the. Water Surface Tension Detergents.
From www.slideshare.net
Soaps and detergents Water Surface Tension Detergents Soap is a commonly used cleaning agent that helps remove dirt and grime from various surfaces. And that's precisely what a surfactant. The reduced surface tension of dishwashing water, and increasing solubility of modern surfactant mixtures, allows the water to run off the dishes in a dish rack very quickly. Detergents lower the surface tension of water. The end of. Water Surface Tension Detergents.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Water Surface Tension Detergents Upon putting a drop of soap solution/detergent on the notch, boat accelerates. To make water wash better, we have to reduce its surface tension so it wets things more uniformly. When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces. Detergents are primarily surfactants, which could be. Water Surface Tension Detergents.
From www.youtube.com
Detergents and Surface Tension YouTube Water Surface Tension Detergents Detergents lower the surface tension of water. Modern detergents contain more than surfactants. The end of the detergent molecule which attaches to fat (grease) repels water molecules. However, the science behind how it. Detergent molecules' two ends make it able to break through the surface tension of water. This series of brief experiments on the surface tension of water, and. Water Surface Tension Detergents.
From shopee.co.th
MizuMi Smooth Cleansing Water Surface Tension Reduction 100/500 ml Water Surface Tension Detergents Soap is a commonly used cleaning agent that helps remove dirt and grime from various surfaces. However, most people also rinse the dishes with pure Upon putting a drop of soap solution/detergent on the notch, boat accelerates. This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as. Water Surface Tension Detergents.
From slideplayer.com
Chapter 10 States of Matter & Water Cycle ppt download Water Surface Tension Detergents Detergent molecules have two ends, one hydrophobic end, repelled by water and one hydrophilic end, attracted to water. Detergents are primarily surfactants, which could be produced easily from petrochemicals. However, the science behind how it. And that's precisely what a surfactant. Modern detergents contain more than surfactants. To make water wash better, we have to reduce its surface tension so. Water Surface Tension Detergents.
From sciencing.com
How Does Detergent Break Surface Tension? Sciencing Water Surface Tension Detergents And that's precisely what a surfactant. Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more likely to interact with oil and grease. However, the science behind how it. Detergents are primarily surfactants, which could be produced easily from petrochemicals. The end of the detergent molecule which. Water Surface Tension Detergents.
From www.slideserve.com
PPT C3.4.14 Detergents PowerPoint Presentation, free download ID Water Surface Tension Detergents Detergents are primarily surfactants, which could be produced easily from petrochemicals. However, the science behind how it. This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to the phenomenon of surface tension,. Surfactants lower the surface tension of water, essentially making it 'wetter' so. Water Surface Tension Detergents.
From es.science19.com
¿Cómo el detergente rompe la tensión superficial? 💫 Portal Multimedia Water Surface Tension Detergents Detergent molecules have two ends, one hydrophobic end, repelled by water and one hydrophilic end, attracted to water. Detergent molecules' two ends make it able to break through the surface tension of water. Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more likely to interact with. Water Surface Tension Detergents.
From www.scribd.com
Surfactants Surface Tension Detergents Wetting Emulsifers Dispersants Water Surface Tension Detergents To make water wash better, we have to reduce its surface tension so it wets things more uniformly. The end of the detergent molecule which attaches to fat (grease) repels water molecules. This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to the phenomenon. Water Surface Tension Detergents.
From www.tutorix.com
Detergents and Surface Tension Water Surface Tension Detergents Detergent molecules' two ends make it able to break through the surface tension of water. Detergents lower the surface tension of water. Modern detergents contain more than surfactants. However, the science behind how it. Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more likely to interact. Water Surface Tension Detergents.
From gtgwithscience.com
3.3 Walking on water the story of surface tension Getting to Grips Water Surface Tension Detergents However, the science behind how it. This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to the phenomenon of surface tension,. Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more. Water Surface Tension Detergents.
From www.instructables.com
How Dish Soap Works Water Surface Tension Experiment 7 Steps (with Water Surface Tension Detergents Detergents lower the surface tension of water. When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces. The end of the detergent molecule which attaches to fat (grease) repels water molecules. However, most people also rinse the dishes with pure The reduced surface tension of dishwashing water,. Water Surface Tension Detergents.
From free-education.in
Surface Tension How detergents work? Angle of Contact Water Surface Tension Detergents However, most people also rinse the dishes with pure To make water wash better, we have to reduce its surface tension so it wets things more uniformly. Detergents are primarily surfactants, which could be produced easily from petrochemicals. Modern detergents contain more than surfactants. When the boat is placed gently on the surface of water, it rests on the surface. Water Surface Tension Detergents.