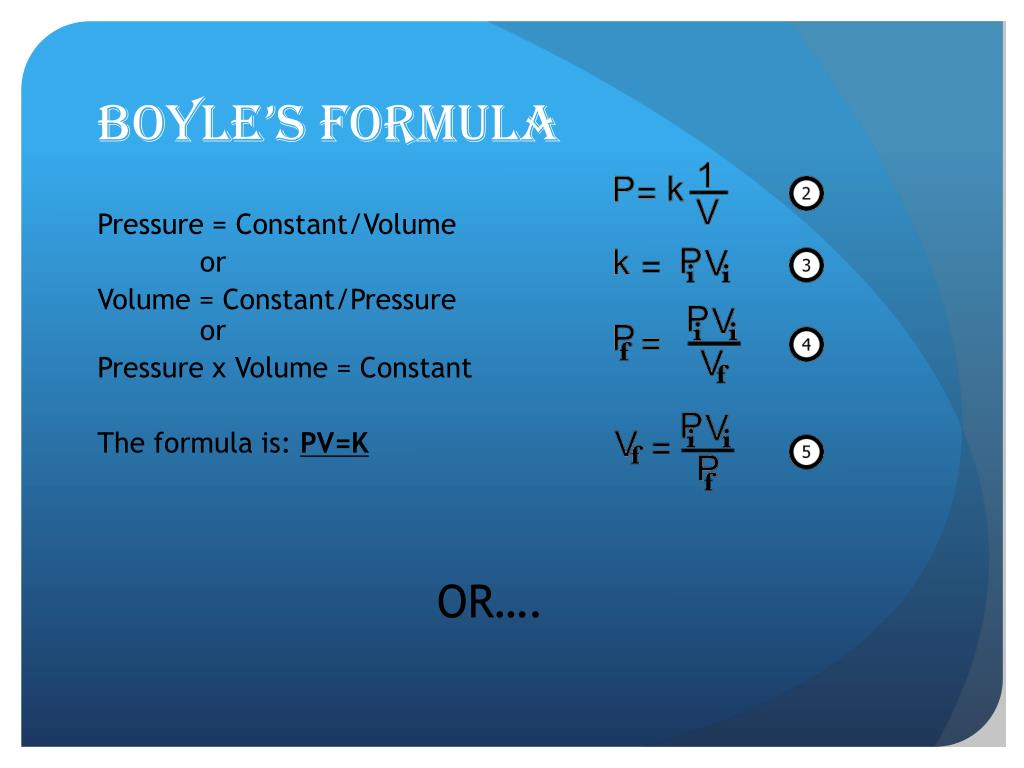
Boyle S Law K Constant . This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: The relationship was also discovered by the french physicist edme mariotte (1676). B o y l e & # 8 2 1 7; A graphical representation of the above equation is shown below. S l a w g r a p h. \[pv = k_{b} \nonumber \] where k a represents a constant value for any given temperature and amount (or mass) of gas. This equation states that the product of pressure and volume is a constant for a given mass confined to a container as long as the temperature remains unchanged. This particular gas law is. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. I.e., in equation form, pv = k, a constant. At constant temperature, the volume of a fixed amount. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas.
from www.slideserve.com
A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. The relationship was also discovered by the french physicist edme mariotte (1676). This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: S l a w g r a p h. I.e., in equation form, pv = k, a constant. B o y l e & # 8 2 1 7; Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. This equation states that the product of pressure and volume is a constant for a given mass confined to a container as long as the temperature remains unchanged. A graphical representation of the above equation is shown below. This particular gas law is.
PPT BOYLE’S LAW PowerPoint Presentation, free download ID5174174
Boyle S Law K Constant S l a w g r a p h. This particular gas law is. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. B o y l e & # 8 2 1 7; This equation states that the product of pressure and volume is a constant for a given mass confined to a container as long as the temperature remains unchanged. A graphical representation of the above equation is shown below. The relationship was also discovered by the french physicist edme mariotte (1676). I.e., in equation form, pv = k, a constant. S l a w g r a p h. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. At constant temperature, the volume of a fixed amount. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: \[pv = k_{b} \nonumber \] where k a represents a constant value for any given temperature and amount (or mass) of gas.
From userenginelumines.z14.web.core.windows.net
Explanation Of Boyle's Law Boyle S Law K Constant S l a w g r a p h. At constant temperature, the volume of a fixed amount. B o y l e & # 8 2 1 7; A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. \[pv = k_{b} \nonumber \] where k a represents a constant. Boyle S Law K Constant.
From scienceforyou.netlify.app
Charles and boyles law scienceforyou Boyle S Law K Constant This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the. Boyle S Law K Constant.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Boyle S Law K Constant A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. \[pv = k_{b} \nonumber \] where k a represents a constant value for any given temperature and amount (or mass) of gas. B o y l e & # 8 2 1 7; Boyle’s law is a gas law which. Boyle S Law K Constant.
From thechemistrynotes.com
Boyle's Law Statement, Formula, Amazing Applications Boyle S Law K Constant The relationship was also discovered by the french physicist edme mariotte (1676). This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v). Boyle S Law K Constant.
From www.chemistrylearner.com
Boyle’s Law Statement, Formula, Graph, and Examples Boyle S Law K Constant The relationship was also discovered by the french physicist edme mariotte (1676). I.e., in equation form, pv = k, a constant. This particular gas law is. This equation states that the product of pressure and volume is a constant for a given mass confined to a container as long as the temperature remains unchanged. This empirical relation, formulated by the. Boyle S Law K Constant.
From www.youtube.com
Boyle's Law (Concepts and Calculations) YouTube Boyle S Law K Constant Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. The relationship was also discovered by the french physicist edme mariotte (1676). S l a w g r a p h. A graphical representation of the above equation is shown. Boyle S Law K Constant.
From cartoondealer.com
Boyle's Law Showing The Pressure And Volume Relationship Vector Boyle S Law K Constant S l a w g r a p h. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: This. Boyle S Law K Constant.
From sciencenotes.org
Boyle's Law Definition, Formula, Example Boyle S Law K Constant This equation states that the product of pressure and volume is a constant for a given mass confined to a container as long as the temperature remains unchanged. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. At constant. Boyle S Law K Constant.
From www.slideserve.com
PPT Boyle’s Law PowerPoint Presentation, free download ID5758944 Boyle S Law K Constant S l a w g r a p h. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: \[pv = k_{b} \nonumber \] where k a represents a constant value for any given temperature and amount (or mass) of gas. This particular gas law is. Boyle’s law is a. Boyle S Law K Constant.
From www.britannica.com
Boyle’s law Definition, Equation, & Facts Britannica Boyle S Law K Constant This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: S l a w g r a p h. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. This. Boyle S Law K Constant.
From www.learnpick.in
Boyle's Law PowerPoint Slides LearnPick India Boyle S Law K Constant The relationship was also discovered by the french physicist edme mariotte (1676). This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; This equation states that the product of pressure and volume is a constant for a given mass. Boyle S Law K Constant.
From studiousguy.com
8 Boyle’s Law Examples in Real Life StudiousGuy Boyle S Law K Constant This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; This particular gas law is. A graphical representation of the above equation is shown below. S l a w g r a p h. Boyle’s law is a gas. Boyle S Law K Constant.
From www.expii.com
GayLussac's Law — Overview & Formula Expii Boyle S Law K Constant This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; This particular gas law is. This equation states that the product of pressure and volume is a constant for a given mass confined to a container as long as. Boyle S Law K Constant.
From www.grc.nasa.gov
Boyle's Law Boyle S Law K Constant Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. \[pv = k_{b} \nonumber \] where k a represents a constant value for any given temperature and amount (or mass) of gas. B o y l e & # 8. Boyle S Law K Constant.
From www.expii.com
Boyle's Law — Overview & Formula Expii Boyle S Law K Constant At constant temperature, the volume of a fixed amount. This particular gas law is. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. S l a w g r a p h. The relationship was also discovered by the. Boyle S Law K Constant.
From www.vecteezy.com
Boyle's law showing that Pressure and volume inversely related in a gas Boyle S Law K Constant This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: A graphical representation of the above equation is shown below. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. \[pv = k_{b} \nonumber \] where k a represents a. Boyle S Law K Constant.
From es.vecteezy.com
de boyle ley, relación Entre presión y volumen de gas a constante Boyle S Law K Constant This particular gas law is. This equation states that the product of pressure and volume is a constant for a given mass confined to a container as long as the temperature remains unchanged. S l a w g r a p h. \[pv = k_{b} \nonumber \] where k a represents a constant value for any given temperature and amount. Boyle S Law K Constant.
From byjus.com
What is the relation between Kh ( Henry's law constant) and molecular Boyle S Law K Constant I.e., in equation form, pv = k, a constant. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of. Boyle S Law K Constant.
From www.slideserve.com
PPT BOYLE’S LAW PowerPoint Presentation, free download ID5174174 Boyle S Law K Constant At constant temperature, the volume of a fixed amount. S l a w g r a p h. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. The relationship was also discovered by the french physicist edme mariotte (1676).. Boyle S Law K Constant.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation, free download ID352441 Boyle S Law K Constant S l a w g r a p h. B o y l e & # 8 2 1 7; This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; A graphical representation of the above equation is shown. Boyle S Law K Constant.
From ar.inspiredpencil.com
Boyles Law Boyle S Law K Constant This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; I.e., in equation form, pv = k, a constant. \[pv = k_{b} \nonumber \] where k a represents a constant value for any given temperature and amount (or mass). Boyle S Law K Constant.
From askfilo.com
Boyle's Law Constant = Mass Constant = Pressure Pressure and Volume are T.. Boyle S Law K Constant This particular gas law is. A graphical representation of the above equation is shown below. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with. Boyle S Law K Constant.
From www.vecteezy.com
Boyle's Law, Relationship between pressure and volume of gas at Boyle S Law K Constant The relationship was also discovered by the french physicist edme mariotte (1676). I.e., in equation form, pv = k, a constant. This particular gas law is. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: At constant temperature, the volume of a fixed amount. This equation states that the. Boyle S Law K Constant.
From eancelblake.blogspot.com
Boyle's Law Data Table EancelBlake Boyle S Law K Constant This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. \[pv = k_{b} \nonumber \] where k a represents a. Boyle S Law K Constant.
From chem.libretexts.org
5.3 The Simple Gas Laws Boyle’s Law, Charles’s Law and Avogadro’s Law Boyle S Law K Constant B o y l e & # 8 2 1 7; A graphical representation of the above equation is shown below. \[pv = k_{b} \nonumber \] where k a represents a constant value for any given temperature and amount (or mass) of gas. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a. Boyle S Law K Constant.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Boyle S Law K Constant Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. B o y l e & # 8 2 1 7; This equation states that the product of pressure and volume is a constant for a given mass confined to. Boyle S Law K Constant.
From solvedlib.com
Use Charles's law to complete table (assume pres… SolvedLib Boyle S Law K Constant This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; This equation states that the product of pressure and volume is a constant for a given mass confined to a container as long as the temperature remains unchanged. This. Boyle S Law K Constant.
From brainly.com
What is an expression of Boyle's law (k = constant)? A. V/T=K B. V = kn Boyle S Law K Constant Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. B o y l e & # 8 2 1 7; I.e., in equation form, pv = k, a constant. A gas law is a simple mathematical formula that allows. Boyle S Law K Constant.
From www.slideserve.com
PPT Unit G PowerPoint Presentation ID552004 Boyle S Law K Constant Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. S l a w g r a p h. I.e., in equation form, pv = k, a constant. A graphical representation of the above equation is shown below. A gas. Boyle S Law K Constant.
From www.expii.com
Charles's Law — Overview & Formula Expii Boyle S Law K Constant A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: I.e., in equation form, pv = k, a constant. \[pv = k_{b} \nonumber \] where k a represents a constant. Boyle S Law K Constant.
From www.pinterest.com
Scientific Robert Boyle is most known for his creation Boyle S Law K Constant A graphical representation of the above equation is shown below. I.e., in equation form, pv = k, a constant. At constant temperature, the volume of a fixed amount. The relationship was also discovered by the french physicist edme mariotte (1676). S l a w g r a p h. A gas law is a simple mathematical formula that allows you. Boyle S Law K Constant.
From www.toppr.com
In Boyle's law what remains constant Boyle S Law K Constant A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. At constant temperature, the volume of a fixed amount. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume. \[pv. Boyle S Law K Constant.
From www.studocu.com
Chapter 5 Questions in Class A Boyle’s Law Problem Example A sample Boyle S Law K Constant This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; This equation states that the product of pressure and volume is a constant for a given mass confined to a container as long as the temperature remains unchanged. The. Boyle S Law K Constant.
From www.numerade.com
SOLVED Potentially Useful Information Dalton'Law of Partial Pressure Boyle S Law K Constant This equation states that the product of pressure and volume is a constant for a given mass confined to a container as long as the temperature remains unchanged. A graphical representation of the above equation is shown below. S l a w g r a p h. A gas law is a simple mathematical formula that allows you to model,. Boyle S Law K Constant.
From www.youtube.com
Boyle's Law Practice 2 YouTube Boyle S Law K Constant At constant temperature, the volume of a fixed amount. The relationship was also discovered by the french physicist edme mariotte (1676). This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: This particular gas law is. This equation states that the product of pressure and volume is a constant for. Boyle S Law K Constant.