Does Chlorine And Oxygen Form An Ionic Compound . There are two chloride ions in the formula. An ionic bond or electrovalent bond is an. Sodium chloride is a compound formed via an ionic bond. Predict the type of compound formed from elements based on their location within the periodic table. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Define ionic and molecular (covalent) compounds. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. A metal (which forms the cations) and a nonmetal. Binary ionic compounds are composed of just two elements: The formation of ionic compounds. An ionic bond is one in which one atom donates an electron to another atom. The charge of the metal ion is determined from the formula of the compound and the charge of the anion.
from emilee-has-crosby.blogspot.com
The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. Sodium chloride is a compound formed via an ionic bond. Predict the type of compound formed from elements based on their location within the periodic table. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. An ionic bond is one in which one atom donates an electron to another atom. An ionic bond or electrovalent bond is an. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. A metal (which forms the cations) and a nonmetal. Define ionic and molecular (covalent) compounds. There are two chloride ions in the formula.
Does Potassium and Magnesium Form an Ionic Compound EmileehasCrosby
Does Chlorine And Oxygen Form An Ionic Compound Binary ionic compounds are composed of just two elements: Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. The formation of ionic compounds. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. An ionic bond is one in which one atom donates an electron to another atom. There are two chloride ions in the formula. Sodium chloride is a compound formed via an ionic bond. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. An ionic bond or electrovalent bond is an. A metal (which forms the cations) and a nonmetal. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. Predict the type of compound formed from elements based on their location within the periodic table. Define ionic and molecular (covalent) compounds. Binary ionic compounds are composed of just two elements:
From brainly.com
Which image depicts the initial atoms when sodium and oxygen form an Does Chlorine And Oxygen Form An Ionic Compound There are two chloride ions in the formula. An ionic bond is one in which one atom donates an electron to another atom. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. An ionic bond or electrovalent bond is an. Sodium chloride is a compound formed via an. Does Chlorine And Oxygen Form An Ionic Compound.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Does Chlorine And Oxygen Form An Ionic Compound Define ionic and molecular (covalent) compounds. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. The formation of ionic compounds. A metal (which forms the cations) and a. Does Chlorine And Oxygen Form An Ionic Compound.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Does Chlorine And Oxygen Form An Ionic Compound Define ionic and molecular (covalent) compounds. There are two chloride ions in the formula. Sodium chloride is a compound formed via an ionic bond. Binary ionic compounds are composed of just two elements: Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. Ions form when atoms lose or gain electrons close electron subatomic particle, with. Does Chlorine And Oxygen Form An Ionic Compound.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Does Chlorine And Oxygen Form An Ionic Compound A metal (which forms the cations) and a nonmetal. An ionic bond or electrovalent bond is an. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. An ionic bond is one in which one atom donates an electron to another atom. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound. Does Chlorine And Oxygen Form An Ionic Compound.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Does Chlorine And Oxygen Form An Ionic Compound Predict the type of compound formed from elements based on their location within the periodic table. The formation of ionic compounds. Define ionic and molecular (covalent) compounds. Binary ionic compounds are composed of just two elements: Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. A metal (which forms the cations) and a nonmetal. An. Does Chlorine And Oxygen Form An Ionic Compound.
From www.gauthmath.com
Solved Magnesium and chlorine react to form an ionic compound. What is Does Chlorine And Oxygen Form An Ionic Compound There are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. Define ionic and molecular (covalent) compounds. Binary ionic compounds are composed of just two elements: The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. An ionic bond or. Does Chlorine And Oxygen Form An Ionic Compound.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Does Chlorine And Oxygen Form An Ionic Compound Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. A metal (which forms the cations) and a nonmetal. An ionic bond or electrovalent bond is an. Sodium chloride is a compound formed via an ionic bond. The charge of the metal ion is determined from the formula of. Does Chlorine And Oxygen Form An Ionic Compound.
From fourthgradegc.blogspot.com
Fourth Grade GC August 2013 Does Chlorine And Oxygen Form An Ionic Compound There are two chloride ions in the formula. Sodium chloride is a compound formed via an ionic bond. Predict the type of compound formed from elements based on their location within the periodic table. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. Ions form when atoms lose or. Does Chlorine And Oxygen Form An Ionic Compound.
From www.thesciencehive.co.uk
Ionic Bonding — the science sauce Does Chlorine And Oxygen Form An Ionic Compound The charge of the metal ion is determined from the formula of the compound and the charge of the anion. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Predict the type of compound formed from elements based on their location within the periodic table. There are two. Does Chlorine And Oxygen Form An Ionic Compound.
From www2.victoriacollege.edu
Chapter 2 Atoms, Molecules and Life Chemistry) Does Chlorine And Oxygen Form An Ionic Compound Define ionic and molecular (covalent) compounds. There are two chloride ions in the formula. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Binary ionic compounds are composed of just two elements: Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. An ionic. Does Chlorine And Oxygen Form An Ionic Compound.
From www.nagwa.com
Question Video Naming Molecular Compound of Chlorine Dioxide Nagwa Does Chlorine And Oxygen Form An Ionic Compound An ionic bond or electrovalent bond is an. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. Define ionic and molecular (covalent) compounds. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. An ionic bond is one in which one atom donates an electron to. Does Chlorine And Oxygen Form An Ionic Compound.
From brainly.com
beryllium be and chlorine cl form a binary ionic compound with a one to Does Chlorine And Oxygen Form An Ionic Compound Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. An ionic bond is one in which one atom donates an electron to another atom. A metal (which forms the cations) and a nonmetal. An ionic bond or electrovalent bond is an. Ions form when atoms lose or gain electrons close electron subatomic particle, with a. Does Chlorine And Oxygen Form An Ionic Compound.
From socratic.org
Chlorine combined with two negative atom or 1 positive and other Does Chlorine And Oxygen Form An Ionic Compound The charge of the metal ion is determined from the formula of the compound and the charge of the anion. Sodium chloride is a compound formed via an ionic bond. Binary ionic compounds are composed of just two elements: Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative.. Does Chlorine And Oxygen Form An Ionic Compound.
From tristianaresshepard.blogspot.com
Oxygen Chemical Formula TristianaresShepard Does Chlorine And Oxygen Form An Ionic Compound A metal (which forms the cations) and a nonmetal. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. There are two chloride ions in the formula. Sodium chloride is a compound formed via an ionic bond. Binary ionic compounds are composed of just two elements: Predict the type of compound. Does Chlorine And Oxygen Form An Ionic Compound.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Does Chlorine And Oxygen Form An Ionic Compound Predict the type of compound formed from elements based on their location within the periodic table. An ionic bond is one in which one atom donates an electron to another atom. Define ionic and molecular (covalent) compounds. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. A metal (which forms. Does Chlorine And Oxygen Form An Ionic Compound.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Does Chlorine And Oxygen Form An Ionic Compound Predict the type of compound formed from elements based on their location within the periodic table. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. An ionic bond or electrovalent bond is an. There are two chloride ions in the formula. An ionic bond is one in which one atom. Does Chlorine And Oxygen Form An Ionic Compound.
From quizizz.com
Properties of Ionic Compounds Chemistry Quiz Quizizz Does Chlorine And Oxygen Form An Ionic Compound An ionic bond is one in which one atom donates an electron to another atom. The formation of ionic compounds. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. An ionic bond or electrovalent bond is an. Define ionic and molecular (covalent) compounds. There are two chloride ions in the formula. The charge of the. Does Chlorine And Oxygen Form An Ionic Compound.
From courses.lumenlearning.com
Lewis Symbols and Structures Chemistry Does Chlorine And Oxygen Form An Ionic Compound A metal (which forms the cations) and a nonmetal. An ionic bond is one in which one atom donates an electron to another atom. Sodium chloride is a compound formed via an ionic bond. The formation of ionic compounds. An ionic bond or electrovalent bond is an. There are two chloride ions in the formula. Although chlorine as an element. Does Chlorine And Oxygen Form An Ionic Compound.
From cartoondealer.com
Ionic Compound Cubic Crystal Structure Cartoon Vector CartoonDealer Does Chlorine And Oxygen Form An Ionic Compound The charge of the metal ion is determined from the formula of the compound and the charge of the anion. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. Predict the type of compound formed from elements based on their location within the periodic table. A metal (which forms the. Does Chlorine And Oxygen Form An Ionic Compound.
From brainly.com
Which image depicts the initial atoms when calcium and sulfur form an Does Chlorine And Oxygen Form An Ionic Compound Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. Binary ionic compounds are composed of just two elements: An ionic bond is one in which one atom donates an electron to another atom. A metal (which forms the cations) and a nonmetal. There are two chloride ions in the formula. An ionic bond or electrovalent. Does Chlorine And Oxygen Form An Ionic Compound.
From brainly.com
Which element when combined with chlorine would most likely form an Does Chlorine And Oxygen Form An Ionic Compound The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. There are two chloride ions in the formula. Predict the type of compound formed from elements based on their location. Does Chlorine And Oxygen Form An Ionic Compound.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Does Chlorine And Oxygen Form An Ionic Compound Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. An ionic bond or electrovalent bond is an. Binary ionic compounds are composed of just two elements: An ionic bond is one in which one atom donates an electron to another atom. The formation of ionic compounds. Ions form when atoms lose or gain electrons close. Does Chlorine And Oxygen Form An Ionic Compound.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Does Chlorine And Oxygen Form An Ionic Compound A metal (which forms the cations) and a nonmetal. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. The formation of ionic compounds. Sodium chloride is a compound formed via an ionic bond. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge. Does Chlorine And Oxygen Form An Ionic Compound.
From www.numerade.com
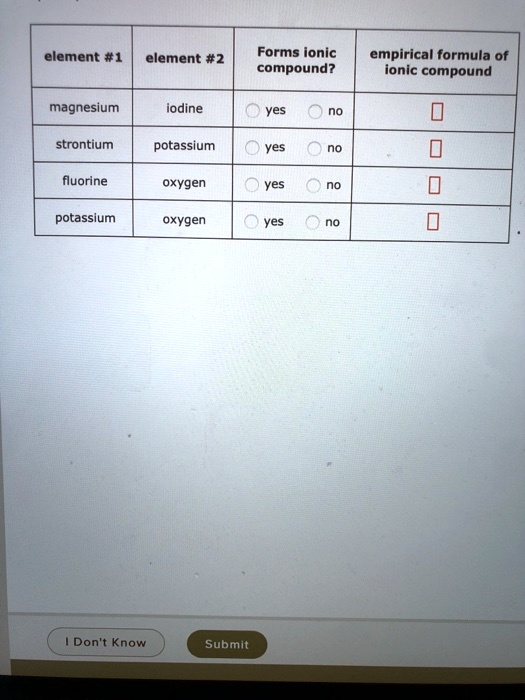
SOLVED empirical Forms ionic formula of ionic compound? compound Does Chlorine And Oxygen Form An Ionic Compound Binary ionic compounds are composed of just two elements: Define ionic and molecular (covalent) compounds. Sodium chloride is a compound formed via an ionic bond. There are two chloride ions in the formula. A metal (which forms the cations) and a nonmetal. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. Predict the type of. Does Chlorine And Oxygen Form An Ionic Compound.
From emilee-has-crosby.blogspot.com
Does Potassium and Magnesium Form an Ionic Compound EmileehasCrosby Does Chlorine And Oxygen Form An Ionic Compound An ionic bond is one in which one atom donates an electron to another atom. Define ionic and molecular (covalent) compounds. Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. Predict the type of compound formed from elements based on their location within the periodic table. The vigorous reaction between the elements sodium and chlorine. Does Chlorine And Oxygen Form An Ionic Compound.
From www.numerade.com
SOLVEDWhich of the following pairs of elements are likely to form an Does Chlorine And Oxygen Form An Ionic Compound The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. Predict the type of compound formed from elements based on their location within the periodic table. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. The charge of the. Does Chlorine And Oxygen Form An Ionic Compound.
From brainly.com
This image shows the ionic bond between sodium and chloride in NaCl Does Chlorine And Oxygen Form An Ionic Compound Sodium chloride is a compound formed via an ionic bond. Binary ionic compounds are composed of just two elements: An ionic bond is one in which one atom donates an electron to another atom. The formation of ionic compounds. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. Define ionic. Does Chlorine And Oxygen Form An Ionic Compound.
From chrisminberger.blogspot.com
What Pairs of Elements Form Ionic Compounds ChrisminBerger Does Chlorine And Oxygen Form An Ionic Compound Predict the type of compound formed from elements based on their location within the periodic table. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. There are two. Does Chlorine And Oxygen Form An Ionic Compound.
From byjus.com
Sodium Chloride Preparation, Properties, Structure & Uses Byju's Does Chlorine And Oxygen Form An Ionic Compound An ionic bond is one in which one atom donates an electron to another atom. There are two chloride ions in the formula. An ionic bond or electrovalent bond is an. Sodium chloride is a compound formed via an ionic bond. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,.. Does Chlorine And Oxygen Form An Ionic Compound.
From www.coursehero.com
[Solved] Decide whether each pair of elements in the table below will Does Chlorine And Oxygen Form An Ionic Compound An ionic bond is one in which one atom donates an electron to another atom. Predict the type of compound formed from elements based on their location within the periodic table. There are two chloride ions in the formula. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt,. Although chlorine. Does Chlorine And Oxygen Form An Ionic Compound.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Does Chlorine And Oxygen Form An Ionic Compound Sodium chloride is a compound formed via an ionic bond. The formation of ionic compounds. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Binary ionic compounds are composed of just two elements: Predict the type of compound formed from elements based on their location within the periodic. Does Chlorine And Oxygen Form An Ionic Compound.
From sciencenotes.org
Ionic Bond Definition and Examples Does Chlorine And Oxygen Form An Ionic Compound There are two chloride ions in the formula. Predict the type of compound formed from elements based on their location within the periodic table. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Does Chlorine And Oxygen Form An Ionic Compound.
From slidesharetrick.blogspot.com
Magnesium And Oxygen Ionic Compound slidesharetrick Does Chlorine And Oxygen Form An Ionic Compound Binary ionic compounds are composed of just two elements: Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. An ionic bond is one in which one atom donates an electron to another atom. A metal (which forms the cations) and a nonmetal. Define ionic and molecular (covalent) compounds.. Does Chlorine And Oxygen Form An Ionic Compound.
From www.nagwa.com
Question Video Determining Which Two Types of Elements Form an Ionic Does Chlorine And Oxygen Form An Ionic Compound Although chlorine as an element is a diatomic molecule, cl 2, elemental chlorine is. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Sodium chloride is a compound formed via an ionic bond. The formation of ionic compounds. An ionic bond is one in which one atom donates. Does Chlorine And Oxygen Form An Ionic Compound.
From www.gauthmath.com
Solved Which image depicts the transfer of electrons between sodium Does Chlorine And Oxygen Form An Ionic Compound Binary ionic compounds are composed of just two elements: The charge of the metal ion is determined from the formula of the compound and the charge of the anion. A metal (which forms the cations) and a nonmetal. An ionic bond is one in which one atom donates an electron to another atom. Define ionic and molecular (covalent) compounds. The. Does Chlorine And Oxygen Form An Ionic Compound.