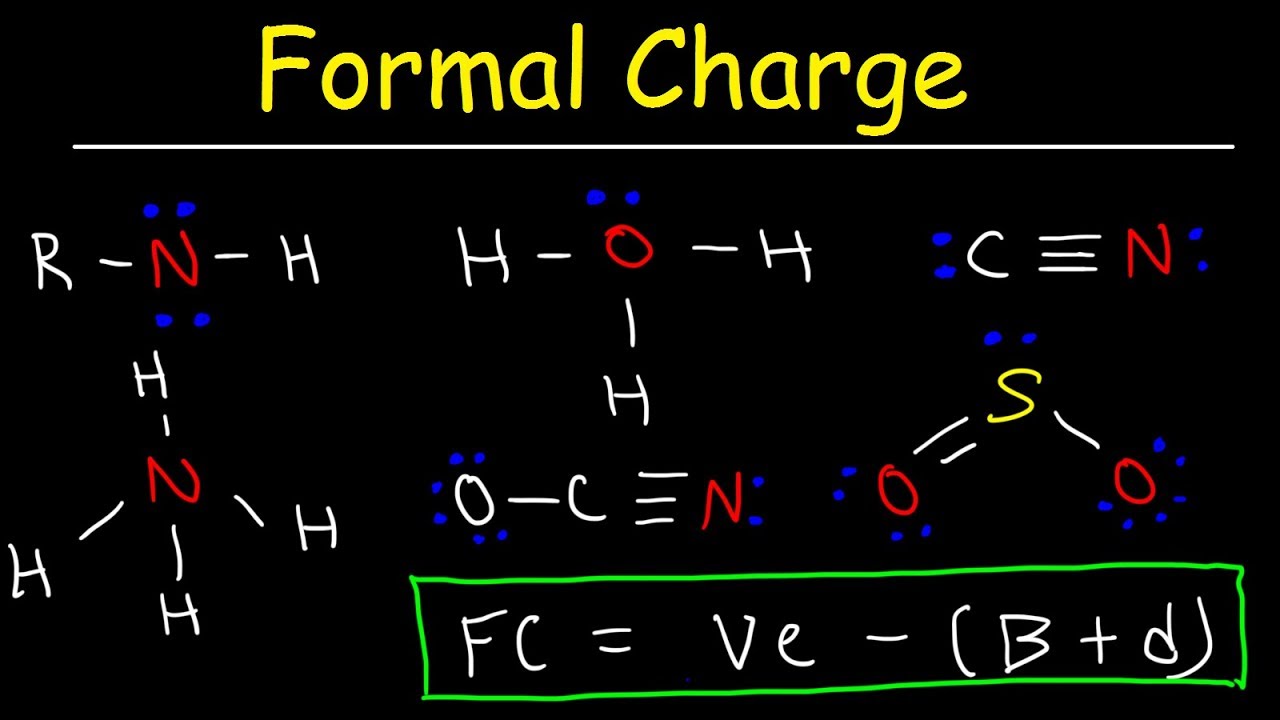
Formal Charge Bromine Atom . Use formal charges to identify the most reasonable lewis structure. Charge is the electrical charge of an atom when all of its ligands are removed homolytically. Compute formal charges for atoms in any lewis structure. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds. In homolytic cleavage, electrons sharing a. In chemistry, a formal charge (f.c. Use formal charges to identify the most reasonable lewis structure for a given molecule. Compute formal charges for atoms in any lewis structure. 93 rows charge (formal charge):
from www.youtube.com
Compute formal charges for atoms in any lewis structure. In homolytic cleavage, electrons sharing a. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical. When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. 93 rows charge (formal charge): Use formal charges to identify the most reasonable lewis structure. In chemistry, a formal charge (f.c. Compute formal charges for atoms in any lewis structure. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds.
How To Calculate The Formal Charge of an Atom Chemistry YouTube
Formal Charge Bromine Atom Compute formal charges for atoms in any lewis structure. Use formal charges to identify the most reasonable lewis structure. Use formal charges to identify the most reasonable lewis structure for a given molecule. In homolytic cleavage, electrons sharing a. In chemistry, a formal charge (f.c. Compute formal charges for atoms in any lewis structure. Compute formal charges for atoms in any lewis structure. Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds. 93 rows charge (formal charge): When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. Charge is the electrical charge of an atom when all of its ligands are removed homolytically.
From www.chegg.com
Solved (5) What is the formal charge on the bromine atom in Formal Charge Bromine Atom 93 rows charge (formal charge): When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. In chemistry, a formal charge (f.c. Compute formal charges for atoms in any lewis structure. Charge is the electrical charge of an atom when all of its ligands are removed homolytically. Compute formal charges. Formal Charge Bromine Atom.
From www.alamy.com
Bromine Atom Shell Stock Vector Image & Art Alamy Formal Charge Bromine Atom A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. 93 rows charge (formal charge): When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. Use formal charges to. Formal Charge Bromine Atom.
From www.collegesearch.in
Formal Charge Formula Definitions, Examples, Significance, Fun Facts Formal Charge Bromine Atom Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical. Compute formal charges for atoms in any lewis structure. 93 rows charge (formal charge): Use formal charges to identify the most reasonable lewis structure for a given molecule. Compute formal charges for atoms in. Formal Charge Bromine Atom.
From byjus.com
What is the oxidation number of bromine in sequence in Br3O8? Formal Charge Bromine Atom Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical. Compute formal charges for atoms in any lewis structure. Compute formal charges for atoms in any lewis structure. When you write lewis structures, you should include formal charges next to each atom with a. Formal Charge Bromine Atom.
From www.numerade.com
SOLVED 1. Which orbital does not house core electrons for a bromine Formal Charge Bromine Atom In chemistry, a formal charge (f.c. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in. Formal Charge Bromine Atom.
From quizdbcornwallis.z21.web.core.windows.net
What Is Bromine Charge Formal Charge Bromine Atom When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds. Compute formal charges for atoms in any lewis structure. Use formal charges to. Formal Charge Bromine Atom.
From www.numerade.com
SOLVED Question 10 of 26 Consider the structure shown. Draw Formal Charge Bromine Atom Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical. Charge is the electrical charge of an atom when all of its ligands are removed homolytically. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a. Formal Charge Bromine Atom.
From www.numerade.com
SOLVED Consider the first Lewis structure for NOBr The formal charge Formal Charge Bromine Atom Compute formal charges for atoms in any lewis structure. Charge is the electrical charge of an atom when all of its ligands are removed homolytically. In homolytic cleavage, electrons sharing a. Use formal charges to identify the most reasonable lewis structure. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule). Formal Charge Bromine Atom.
From washedupcelebrity.blogspot.com
Draw The Product For The Following Reaction Including Formal Charge If Formal Charge Bromine Atom 93 rows charge (formal charge): In chemistry, a formal charge (f.c. Charge is the electrical charge of an atom when all of its ligands are removed homolytically. Compute formal charges for atoms in any lewis structure. Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in. Formal Charge Bromine Atom.
From studyschoolinterlock.z21.web.core.windows.net
Calculating Formal Charge Practice Formal Charge Bromine Atom Use formal charges to identify the most reasonable lewis structure for a given molecule. Compute formal charges for atoms in any lewis structure. In homolytic cleavage, electrons sharing a. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds. Compute formal charges for atoms. Formal Charge Bromine Atom.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Formal Charge Bromine Atom When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds. A formal charge (\(fc\)) compares the number of electrons around a neutral atom. Formal Charge Bromine Atom.
From facts.net
18 Captivating Facts About Formal Charge Formal Charge Bromine Atom Use formal charges to identify the most reasonable lewis structure for a given molecule. 93 rows charge (formal charge): A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. In homolytic cleavage, electrons sharing a. In chemistry, a formal charge. Formal Charge Bromine Atom.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Formal Charge Bromine Atom 93 rows charge (formal charge): Charge is the electrical charge of an atom when all of its ligands are removed homolytically. In homolytic cleavage, electrons sharing a. Compute formal charges for atoms in any lewis structure. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons. Formal Charge Bromine Atom.
From app.emaze.com
Bromine ) on emaze Formal Charge Bromine Atom A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds. In chemistry, a formal. Formal Charge Bromine Atom.
From www.dreamstime.com
Bromine Atom, with Mass and Energy Levels. Stock Vector Illustration Formal Charge Bromine Atom Compute formal charges for atoms in any lewis structure. Use formal charges to identify the most reasonable lewis structure. 93 rows charge (formal charge): Compute formal charges for atoms in any lewis structure. When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. Charge is the electrical charge of. Formal Charge Bromine Atom.
From mungfali.com
Bromine Electron Dot Diagram Formal Charge Bromine Atom Use formal charges to identify the most reasonable lewis structure. When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical. A formal charge. Formal Charge Bromine Atom.
From www.coursehero.com
[Solved] The formal charge on the bromine atom in BrO 3 drawn with Formal Charge Bromine Atom Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical. Compute formal charges for atoms in any lewis structure. 93 rows charge (formal charge): Use formal charges to identify the most reasonable lewis structure. The formal charge of an atom in a molecule is. Formal Charge Bromine Atom.
From quizdbcornwallis.z21.web.core.windows.net
What Charge Is Bromine Formal Charge Bromine Atom In chemistry, a formal charge (f.c. Charge is the electrical charge of an atom when all of its ligands are removed homolytically. Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical. Compute formal charges for atoms in any lewis structure. Use formal charges. Formal Charge Bromine Atom.
From ar.inspiredpencil.com
Bromine Pentafluoride Formal Charge Formal Charge Bromine Atom Charge is the electrical charge of an atom when all of its ligands are removed homolytically. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. 93 rows charge (formal charge): Compute formal charges for atoms in any lewis structure.. Formal Charge Bromine Atom.
From www.numerade.com
(a Determine the formal charge on the bromine atom in the following Formal Charge Bromine Atom Use formal charges to identify the most reasonable lewis structure for a given molecule. 93 rows charge (formal charge): A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. Compute formal charges for atoms in any lewis structure. When you. Formal Charge Bromine Atom.
From www.chegg.com
Solved Determine the formal charge on the bromine atom in Formal Charge Bromine Atom In homolytic cleavage, electrons sharing a. Use formal charges to identify the most reasonable lewis structure for a given molecule. Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical. 93 rows charge (formal charge): The formal charge of an atom in a molecule. Formal Charge Bromine Atom.
From www.numerade.com
SOLVED Draw the best Lewis structure for BrO4 and determine the formal Formal Charge Bromine Atom Use formal charges to identify the most reasonable lewis structure. 93 rows charge (formal charge): In chemistry, a formal charge (f.c. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds. When you write lewis structures, you should include formal charges next to each. Formal Charge Bromine Atom.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube Formal Charge Bromine Atom In chemistry, a formal charge (f.c. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds. Compute formal charges for atoms in any lewis structure. Use formal charges to identify the most reasonable lewis structure. Or q*), in the covalent view of chemical bonding,. Formal Charge Bromine Atom.
From hanenhuusholli.blogspot.com
Lewis Dot Diagram For Bromine Hanenhuusholli Formal Charge Bromine Atom Use formal charges to identify the most reasonable lewis structure for a given molecule. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. In homolytic cleavage, electrons sharing a. Use formal charges to identify the most reasonable lewis structure.. Formal Charge Bromine Atom.
From www.coursehero.com
[Solved] b 0 00 Br 0in A. What is the formal charge on the Formal Charge Bromine Atom Compute formal charges for atoms in any lewis structure. Charge is the electrical charge of an atom when all of its ligands are removed homolytically. Use formal charges to identify the most reasonable lewis structure. When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. 93 rows charge (formal. Formal Charge Bromine Atom.
From www.numerade.com
How many covalent bonds will be drawn to bromine in BrO for the dot Formal Charge Bromine Atom Use formal charges to identify the most reasonable lewis structure for a given molecule. In chemistry, a formal charge (f.c. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. Use formal charges to identify the most reasonable lewis structure.. Formal Charge Bromine Atom.
From www.youtube.com
How To Calculate The Formal Charge of an Atom Chemistry YouTube Formal Charge Bromine Atom Charge is the electrical charge of an atom when all of its ligands are removed homolytically. Compute formal charges for atoms in any lewis structure. Compute formal charges for atoms in any lewis structure. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an. Formal Charge Bromine Atom.
From www.numerade.com
SOLVED Draw the best Lewis structure for Cl3" What is the formal Formal Charge Bromine Atom Use formal charges to identify the most reasonable lewis structure. In homolytic cleavage, electrons sharing a. Compute formal charges for atoms in any lewis structure. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. Or q*), in the covalent. Formal Charge Bromine Atom.
From wisc.pb.unizin.org
Resonance Structures and Formal Charge (M8Q3) UWMadison Chemistry Formal Charge Bromine Atom A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. Compute formal charges for atoms in any lewis structure. 93 rows charge (formal charge): In chemistry, a formal charge (f.c. Charge is the electrical charge of an atom when all. Formal Charge Bromine Atom.
From www.chegg.com
Solved The formal charge on the bromine atom in BrO3−drawn Formal Charge Bromine Atom Use formal charges to identify the most reasonable lewis structure for a given molecule. Compute formal charges for atoms in any lewis structure. In homolytic cleavage, electrons sharing a. Compute formal charges for atoms in any lewis structure. Use formal charges to identify the most reasonable lewis structure. Or q*), in the covalent view of chemical bonding, is the hypothetical. Formal Charge Bromine Atom.
From www.numerade.com
SOLVED A Lewis structure with placeholder elements is shown below. If Formal Charge Bromine Atom In chemistry, a formal charge (f.c. Charge is the electrical charge of an atom when all of its ligands are removed homolytically. 93 rows charge (formal charge): When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. Use formal charges to identify the most reasonable lewis structure. Or q*),. Formal Charge Bromine Atom.
From www.numerade.com
SOLVED Draw the best (most probable) Lewis structure for BrO and Formal Charge Bromine Atom Use formal charges to identify the most reasonable lewis structure. In homolytic cleavage, electrons sharing a. A formal charge (\(fc\)) compares the number of electrons around a neutral atom (an atom not in a molecule) versus the number of electrons around an atom in a molecule. Charge is the electrical charge of an atom when all of its ligands are. Formal Charge Bromine Atom.
From www.numerade.com
(a Determine the formal charge on the bromine atom in the following Formal Charge Bromine Atom When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds. In homolytic cleavage, electrons sharing a. Or q*), in the covalent view of. Formal Charge Bromine Atom.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Formal Charge Bromine Atom When you write lewis structures, you should include formal charges next to each atom with a formal charge that isn't 0. Use formal charges to identify the most reasonable lewis structure for a given molecule. Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all. Formal Charge Bromine Atom.
From www.chegg.com
Solved The formal charge on the bromine atom in BrO3−drawn Formal Charge Bromine Atom The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds. Or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical. Compute formal charges for atoms in any lewis. Formal Charge Bromine Atom.