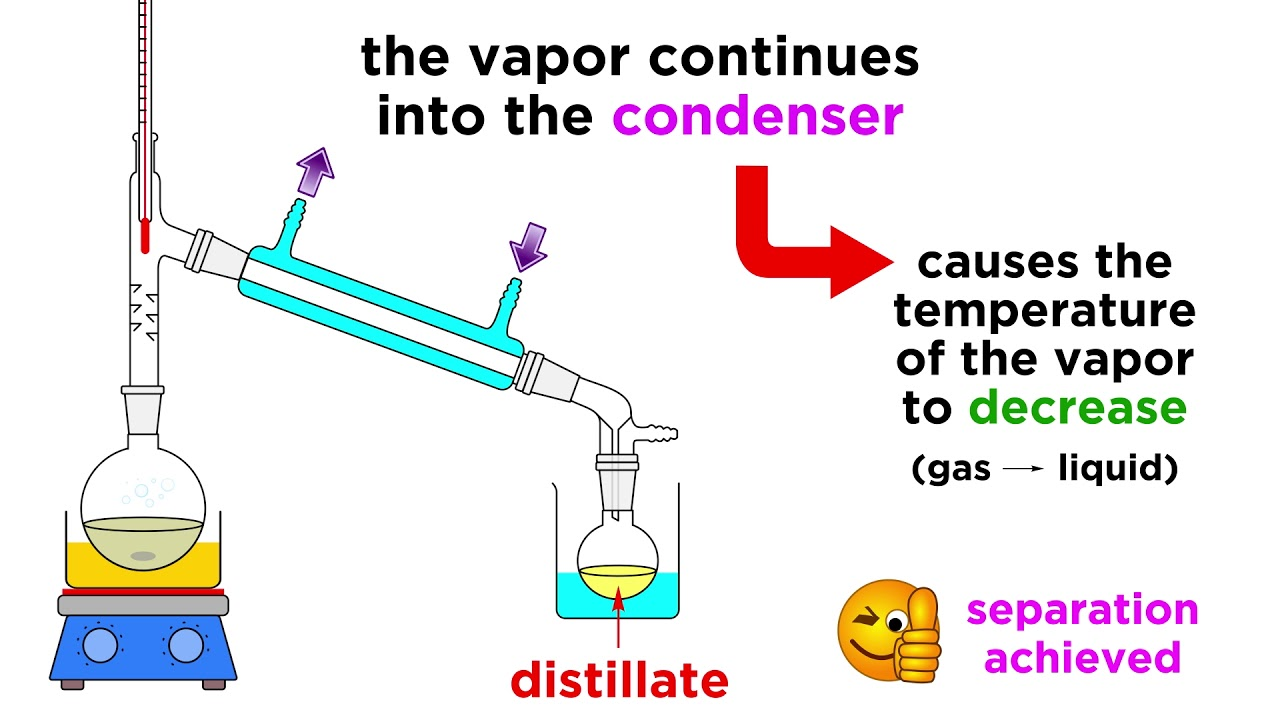
Purification Chemistry Meaning . Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. The point has been to give an overview of the key techniques used for purifying crude mixtures: The choice of separation technique depends on the substances being separated. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. All techniques rely on a. Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. There are different ways to separate mixtures, for.
from www.smacgigworld.com
The choice of separation technique depends on the substances being separated. There are different ways to separate mixtures, for. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. The point has been to give an overview of the key techniques used for purifying crude mixtures: Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. All techniques rely on a.
Laboratory Water Purification Technologies and Their Advantages
Purification Chemistry Meaning All techniques rely on a. The point has been to give an overview of the key techniques used for purifying crude mixtures: Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. All techniques rely on a. There are different ways to separate mixtures, for. Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. The choice of separation technique depends on the substances being separated. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc.
From www.youtube.com
Pronunciation of Purification Definition of Purification YouTube Purification Chemistry Meaning There are different ways to separate mixtures, for. The point has been to give an overview of the key techniques used for purifying crude mixtures: Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. Separations can be achieved by differences in physical properties, such as. Purification Chemistry Meaning.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记7.6.5 Organic Techniques Purification Purification Chemistry Meaning Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. The point has been to give an overview of the key techniques used for purifying crude mixtures: The choice of separation technique depends on the substances being separated. Purification in a chemical context is the physical. Purification Chemistry Meaning.
From www.slideserve.com
PPT WATER PURIFICATION PowerPoint Presentation, free download ID Purification Chemistry Meaning Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. All techniques rely on a. The choice of separation technique depends on the substances being separated. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. Purification. Purification Chemistry Meaning.
From www.tes.com
Chemistry Required Practical 8 Water purification Teaching Resources Purification Chemistry Meaning The point has been to give an overview of the key techniques used for purifying crude mixtures: 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. Purification in a chemical context is the physical. Purification Chemistry Meaning.
From ar.inspiredpencil.com
Sublimation Method Of Separation Purification Chemistry Meaning The choice of separation technique depends on the substances being separated. The point has been to give an overview of the key techniques used for purifying crude mixtures: Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. Separations can be achieved by differences in physical. Purification Chemistry Meaning.
From www.youtube.com
METHODS OF PURIFICATION OF ORGANIC COMPOUNDS YouTube Purification Chemistry Meaning Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. Purification is very important to chemistry as it allows for the reagents and organic compounds. Purification Chemistry Meaning.
From www.youtube.com
PURIFICATION OF WATER PURIFICATION METHODS SCIENCE EDUCATIONAL Purification Chemistry Meaning Here’s a handy table summarizing the advantages and disadvantages of each for each scale. Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. There are different ways to separate mixtures, for. Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical.. Purification Chemistry Meaning.
From pdfprof.com
extraction et purification des protéines Purification Chemistry Meaning All techniques rely on a. Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. There are different ways to separate mixtures, for. The point has been to give an overview of the key techniques used for purifying crude. Purification Chemistry Meaning.
From www.vedantu.com
What do you mean by filtration. Explain with a diagram. Purification Chemistry Meaning Here’s a handy table summarizing the advantages and disadvantages of each for each scale. Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. There are different ways to separate mixtures, for. The point has been to give an overview of the key techniques used for. Purification Chemistry Meaning.
From www.youtube.com
Purification meaning of Purification YouTube Purification Chemistry Meaning There are different ways to separate mixtures, for. Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. The choice of. Purification Chemistry Meaning.
From www.chemicals.co.uk
GCSE Chemistry Water Purification Guide The Chemistry Blog Purification Chemistry Meaning The point has been to give an overview of the key techniques used for purifying crude mixtures: Here’s a handy table summarizing the advantages and disadvantages of each for each scale. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. There are different ways to separate mixtures, for. Separations can be achieved by differences in physical properties, such. Purification Chemistry Meaning.
From waterfilterspot.com
How To Purify Water The Different Water Purification Methods Available! Purification Chemistry Meaning Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. There are different ways to separate mixtures, for. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. Purification in a chemical context is the physical separation. Purification Chemistry Meaning.
From yourechemistry.blogspot.com
Just Chemistry The techniques Purification Chemistry Meaning The choice of separation technique depends on the substances being separated. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. The point has been to give an overview of the key techniques used for purifying crude mixtures: All techniques rely on a. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. There are. Purification Chemistry Meaning.
From classnotes.org.in
Purification of Organic Compounds Chemistry, Class 11, Organic Purification Chemistry Meaning Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. There are different ways to separate mixtures, for. The point has been to give an overview. Purification Chemistry Meaning.
From www.aakash.ac.in
Crystallization Process, Precautions, Advantages & Examples Purification Chemistry Meaning Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. There are different ways to separate mixtures, for. Separations can be achieved by differences in physical. Purification Chemistry Meaning.
From www.slideshare.net
Purification Of Substances 1 Purification Chemistry Meaning There are different ways to separate mixtures, for. The point has been to give an overview of the key techniques used for purifying crude mixtures: 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. The choice of separation technique depends on the substances being separated. All techniques rely on a. Here’s a handy table summarizing the advantages and. Purification Chemistry Meaning.
From www.slideshare.net
Purification Of Substances 1 Purification Chemistry Meaning Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. The choice. Purification Chemistry Meaning.
From www.vecteezy.com
Filtration process of mixture of solid and liquid science experiment Purification Chemistry Meaning Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. The point has been to give an overview of the key. Purification Chemistry Meaning.
From www.mdpi.com
Materials Free FullText Recent Achievements in Polymer BioBased Purification Chemistry Meaning The choice of separation technique depends on the substances being separated. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. All techniques rely on a. Purification is very important to chemistry as it allows for the reagents and organic compounds. Purification Chemistry Meaning.
From www.slideserve.com
PPT Methods of Purification PowerPoint Presentation, free download Purification Chemistry Meaning All techniques rely on a. Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. The choice of separation technique depends on the substances being separated. The point has been to give an overview of the key techniques used for purifying crude mixtures: Separations can be achieved by differences in. Purification Chemistry Meaning.
From www.youtube.com
Water Purification Methods Water Purification How to Make Water Purification Chemistry Meaning Here’s a handy table summarizing the advantages and disadvantages of each for each scale. All techniques rely on a. Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. There are different ways to separate mixtures, for. The point has been to give an overview of the key techniques used for purifying. Purification Chemistry Meaning.
From www.smacgigworld.com
Laboratory Water Purification Technologies and Their Advantages Purification Chemistry Meaning 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. All techniques rely on a. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. There are different ways to separate mixtures, for. The point has been. Purification Chemistry Meaning.
From deliverynews.weebly.com
Crystallization Of Organic Compounds deliverynews Purification Chemistry Meaning The point has been to give an overview of the key techniques used for purifying crude mixtures: Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. There are different ways to separate mixtures, for. Separations can be achieved by differences in physical properties, such as. Purification Chemistry Meaning.
From classnotes.org.in
Purification of Organic Compounds Chemistry, Class 11, Organic Purification Chemistry Meaning There are different ways to separate mixtures, for. Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. All techniques rely on a. Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. Here’s a handy table summarizing. Purification Chemistry Meaning.
From www.slideshare.net
Purification Of Substances 1 Purification Chemistry Meaning Here’s a handy table summarizing the advantages and disadvantages of each for each scale. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. The point has been to give an overview of the key techniques used for purifying crude mixtures: Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical.. Purification Chemistry Meaning.
From comis.med.uvm.edu
Protein Purification Methods Purification Chemistry Meaning All techniques rely on a. Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. The point has been to give. Purification Chemistry Meaning.
From www.askmattrab.com
Purification or Refining of Metals Class Eleven Chemistry Purification Chemistry Meaning There are different ways to separate mixtures, for. The point has been to give an overview of the key techniques used for purifying crude mixtures: All techniques rely on a. Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. The choice of separation technique depends on the substances being. Purification Chemistry Meaning.
From www.scribd.com
Separation and Purification in Chemistry Grade 9 Chromatography Purification Chemistry Meaning The choice of separation technique depends on the substances being separated. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. All techniques rely on a. The point has been to give an overview of the key techniques used for purifying crude mixtures: Purification in a chemical context is the physical separation of a chemical substance of interest from. Purification Chemistry Meaning.
From fr.dreamstime.com
Water Purification System With Labeled Filtration Stages Outline Purification Chemistry Meaning There are different ways to separate mixtures, for. Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. The point has been to give an overview of the key techniques used for purifying crude mixtures: All techniques rely on a. Purification is very important to chemistry as it allows for the reagents. Purification Chemistry Meaning.
From www.chemicals.co.uk
GCSE Chemistry Water Purification Guide The Chemistry Blog Purification Chemistry Meaning Here’s a handy table summarizing the advantages and disadvantages of each for each scale. Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. The choice of separation technique depends on the substances being separated. Separations can be achieved by differences in physical properties, such as differences in boiling point,. Purification Chemistry Meaning.
From vadic.vigyanashram.blog
Assignment Recrystallization of Oxalic Acid DESIGN INNOVATION CENTER Purification Chemistry Meaning Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. The point has been to give an overview of the key techniques used for purifying crude mixtures: Here’s a handy table summarizing the advantages and disadvantages of each for each scale. 1) chemical properties, 2) distillation,. Purification Chemistry Meaning.
From www.slideshare.net
Water purification methods Purification Chemistry Meaning 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. Here’s a handy table summarizing. Purification Chemistry Meaning.
From byjus.com
Crystallisation Questions Practice Questions of Crystallisation with Purification Chemistry Meaning Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. Here’s a handy table summarizing the advantages and disadvantages of each for each scale. Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity for reactions. There are. Purification Chemistry Meaning.
From www.youtube.com
Purification Meaning YouTube Purification Chemistry Meaning Here’s a handy table summarizing the advantages and disadvantages of each for each scale. All techniques rely on a. There are different ways to separate mixtures, for. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. Purification is very important to chemistry as it allows for the reagents and organic compounds we use to be of high purity. Purification Chemistry Meaning.
From ar.inspiredpencil.com
Sublimation Examples Chemistry Purification Chemistry Meaning Purification in a chemical context is the physical separation of a chemical substance of interest from foreign or contaminating substances. Separations can be achieved by differences in physical properties, such as differences in boiling point, or by chemical. 1) chemical properties, 2) distillation, 3) crystallization, 4) chromatography, 5) gc/hplc. Purification is very important to chemistry as it allows for the. Purification Chemistry Meaning.