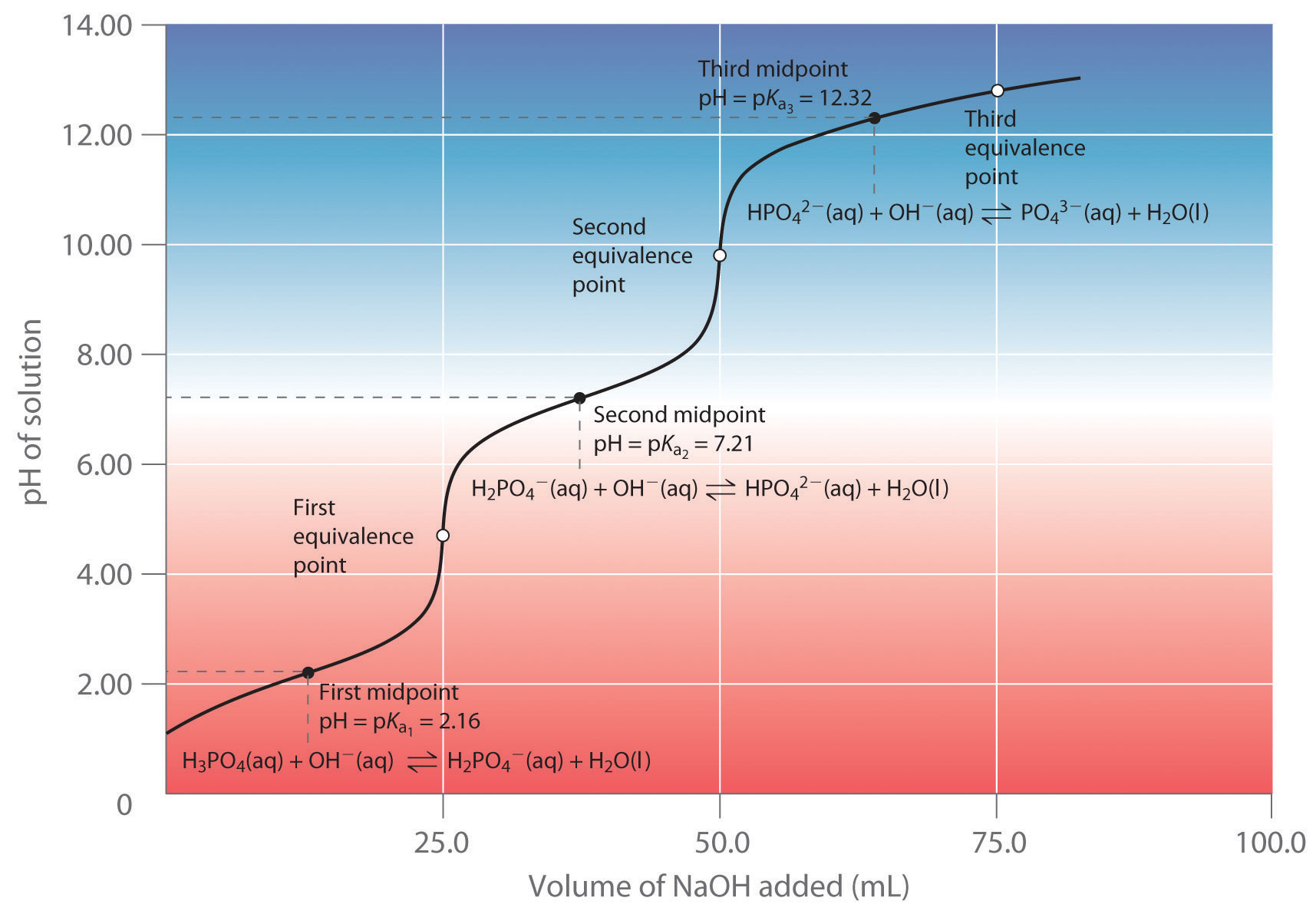
Acid Base Titration Naoh . The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). Titration is a common laboratory technique for determining the concentration of a solute. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. An indicator can be added to show. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these.
from saylordotorg.github.io
A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. An indicator can be added to show. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). Titration is a common laboratory technique for determining the concentration of a solute. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these.
AcidBase Titrations
Acid Base Titration Naoh In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). An indicator can be added to show. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Titration is a common laboratory technique for determining the concentration of a solute.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Acid Base Titration Naoh The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). Titration is a common laboratory technique for determining the concentration of a solute. A titration is carried out for 25.00 ml of 0.100 m hcl. Acid Base Titration Naoh.
From www.careerstoday.in
Acid Base Titration Careers Today Acid Base Titration Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Titration is a common laboratory technique for determining the concentration of a solute. An indicator can be added to show. In this section we will learn how to calculate the ph. Acid Base Titration Naoh.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Acid Base Titration Naoh An indicator can be added to show. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of. Acid Base Titration Naoh.
From saylordotorg.github.io
AcidBase Titrations Acid Base Titration Naoh The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. An indicator can be. Acid Base Titration Naoh.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Acid Base Titration Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. An indicator can be added to show. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Titration is a. Acid Base Titration Naoh.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Acid Base Titration Naoh In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Titration is a common laboratory technique for determining the concentration. Acid Base Titration Naoh.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid Base Titration Naoh The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. An indicator can be. Acid Base Titration Naoh.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Acid Base Titration Naoh An indicator can be added to show. Titration is a common laboratory technique for determining the concentration of a solute. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. In this section we will learn how to calculate the ph. Acid Base Titration Naoh.
From www.slideshare.net
Acid base titration (1) Acid Base Titration Naoh The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). An indicator can be added to show. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of. Acid Base Titration Naoh.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Acid Base Titration Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration. Acid Base Titration Naoh.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Acid Base Titration Naoh The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. Acid Base Titration Naoh.
From www.animalia-life.club
Acid Base Chemistry Acid Base Titration Naoh In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Titration is a common laboratory technique for determining the concentration of a solute. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. Acid Base Titration Naoh.
From studylib.net
AcidBase Titration Answers, Sources of Error Acid Base Titration Naoh In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). An indicator can be. Acid Base Titration Naoh.
From www.chegg.com
Solved Summary Data Table AcidBase Titration CHEZ 101L Acid Base Titration Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration. Acid Base Titration Naoh.
From www.myxxgirl.com
What Is Double Titration Theory By Using Sulphuric Acid My XXX Hot Girl Acid Base Titration Naoh In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Titration is a common laboratory technique for determining the concentration of a solute. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. Acid Base Titration Naoh.
From mungfali.com
Acid Base Titration Calculation Acid Base Titration Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration. Acid Base Titration Naoh.
From webmis.highland.cc.il.us
AcidBase Titrations Acid Base Titration Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration. Acid Base Titration Naoh.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Acid Base Titration Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Titration is a common laboratory technique for determining the concentration of a solute. An indicator can be added to show. In this section we will learn how to calculate the ph. Acid Base Titration Naoh.
From saylordotorg.github.io
AcidBase Titrations Acid Base Titration Naoh An indicator can be added to show. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Titration is a common laboratory technique for determining the concentration of a solute. In this section we will learn how to calculate the ph. Acid Base Titration Naoh.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Acid Base Titration Naoh Titration is a common laboratory technique for determining the concentration of a solute. An indicator can be added to show. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The graph shows a titration curve for the titration of 25.00. Acid Base Titration Naoh.
From mungfali.com
HCl NaOH Titration Acid Base Titration Naoh The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. A titration is carried. Acid Base Titration Naoh.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Acid Base Titration Naoh An indicator can be added to show. Titration is a common laboratory technique for determining the concentration of a solute. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). In this section we will. Acid Base Titration Naoh.
From www.sliderbase.com
Titration Acid Base Titration Naoh An indicator can be added to show. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of. Acid Base Titration Naoh.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Acid Base Titration Naoh Titration is a common laboratory technique for determining the concentration of a solute. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. An indicator can be added to show. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a. Acid Base Titration Naoh.
From saylordotorg.github.io
AcidBase Titrations Acid Base Titration Naoh In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. An indicator can be added to show. Titration is a common laboratory technique for determining the concentration of a solute. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a. Acid Base Titration Naoh.
From dokumen.tips
(PDF) AcidBase Titration NaOH with HCL DOKUMEN.TIPS Acid Base Titration Naoh Titration is a common laboratory technique for determining the concentration of a solute. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. In this section we will learn how to calculate the ph of an analyte solution throughout the titration,. Acid Base Titration Naoh.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Acid Base Titration Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Titration is a common laboratory technique for determining the concentration of a solute. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid). Acid Base Titration Naoh.
From mungfali.com
Acid Base Titration Calculation Acid Base Titration Naoh In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Titration is a common laboratory technique for determining the concentration of a solute. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. Acid Base Titration Naoh.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Acid Base Titration Naoh Titration is a common laboratory technique for determining the concentration of a solute. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid). Acid Base Titration Naoh.
From www.vrogue.co
Solved Acid Base Titrations Consider The Titration Of vrogue.co Acid Base Titration Naoh Titration is a common laboratory technique for determining the concentration of a solute. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). In this section we will learn how to calculate the ph of. Acid Base Titration Naoh.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Acid Base Titration Naoh In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). Titration is a common. Acid Base Titration Naoh.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Acid Base Titration Naoh In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). Titration is a common. Acid Base Titration Naoh.
From www.youtube.com
Lab Demonstration Acid Base Titration. YouTube Acid Base Titration Naoh In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of hcl (strong acid). An indicator can be. Acid Base Titration Naoh.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Acid Base Titration Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration. Acid Base Titration Naoh.
From www.vrogue.co
Standardization Of Naoh Acid Base Titration Objective vrogue.co Acid Base Titration Naoh An indicator can be added to show. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The graph shows a titration curve for the titration of 25.00 ml of 0.100 m ch3co2h (weak acid) with 0.100 m naoh (strong base) and the titration curve for the titration of. Acid Base Titration Naoh.