Two Uses Of Titration In Chemistry . Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. When an acid and alkali react together, a neutralisation. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. It is commonly used in analytical chemistry to. Perform and interpret titration calculations. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated.
from boisestate.pressbooks.pub
A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. When an acid and alkali react together, a neutralisation. It is commonly used in analytical chemistry to. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Perform and interpret titration calculations. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance.
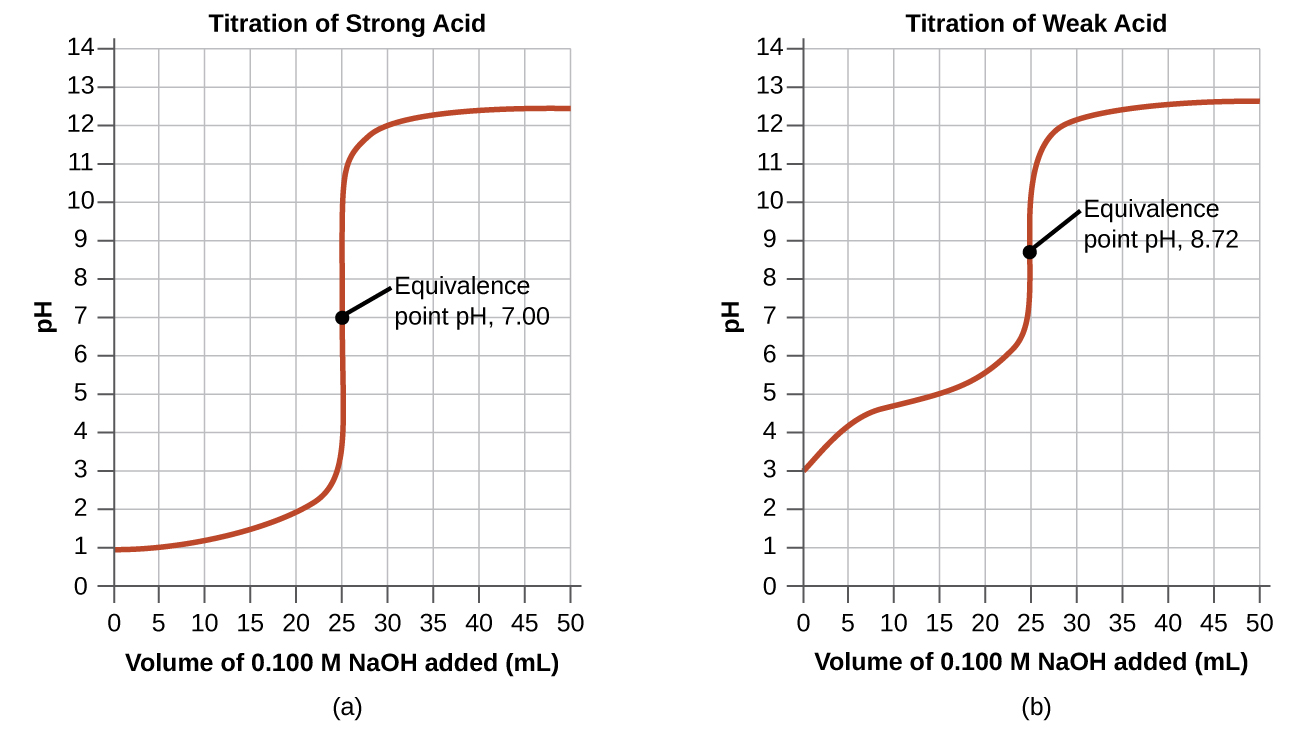
14.7 AcidBase Titrations General Chemistry 1 & 2
Two Uses Of Titration In Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is commonly used in analytical chemistry to. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Perform and interpret titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. When an acid and alkali react together, a neutralisation.
From www.vrogue.co
14 3 Redox Reactions And Titrations Chemistry Librete vrogue.co Two Uses Of Titration In Chemistry When an acid and alkali react together, a neutralisation. Perform and interpret titration calculations. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a. Two Uses Of Titration In Chemistry.
From quizizz.com
Titration Method Chemistry Quiz Quizizz Two Uses Of Titration In Chemistry It is commonly used in analytical chemistry to. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. Titration is the slow addition of. Two Uses Of Titration In Chemistry.
From giodaqksz.blob.core.windows.net
Acid Base Titration Guide at Mary Mcgee blog Two Uses Of Titration In Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Perform and interpret titration calculations. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration (also known as titrimetry [1] and volumetric analysis) is a. Two Uses Of Titration In Chemistry.
From boardtopper.blogspot.com
Titration 1 Class 12 CBSE Chemistry Practical All Study Guide at Two Uses Of Titration In Chemistry Perform and interpret titration calculations. It is commonly used in analytical chemistry to. When an acid and alkali react together, a neutralisation. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated.. Two Uses Of Titration In Chemistry.
From gioxaqmhp.blob.core.windows.net
Indicators Titration at Lee Hughes blog Two Uses Of Titration In Chemistry It is commonly used in analytical chemistry to. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. Perform and interpret titration calculations. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. A. Two Uses Of Titration In Chemistry.
From buoiholo.edu.vn
รายการ 105+ ภาพ Titrate ทางการแพทย์ ใหม่ที่สุด Two Uses Of Titration In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Perform and interpret titration calculations. When an acid and alkali react together, a neutralisation. It is. Two Uses Of Titration In Chemistry.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Two Uses Of Titration In Chemistry When an acid and alkali react together, a neutralisation. Perform and interpret titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Titration is a widely used technique to measure the. Two Uses Of Titration In Chemistry.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Two Uses Of Titration In Chemistry A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. When an acid and alkali react together, a neutralisation. It is commonly used in. Two Uses Of Titration In Chemistry.
From www.vrogue.co
Gcse Chemistry Titration Calculations Teaching Resources www.vrogue.co Two Uses Of Titration In Chemistry When an acid and alkali react together, a neutralisation. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Two Uses Of Titration In Chemistry.
From www.savemyexams.co.uk
Titrations (1.7.2) Edexcel A Level Chemistry Revision Notes 2017 Two Uses Of Titration In Chemistry When an acid and alkali react together, a neutralisation. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. Perform and interpret titration. Two Uses Of Titration In Chemistry.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Two Uses Of Titration In Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. It is commonly used in analytical chemistry to. Titration is a widely used. Two Uses Of Titration In Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Two Uses Of Titration In Chemistry A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. It is commonly used in analytical chemistry to. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method. Two Uses Of Titration In Chemistry.
From theedge.com.hk
Chemistry How To Titration The Edge Two Uses Of Titration In Chemistry A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a. Two Uses Of Titration In Chemistry.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Two Uses Of Titration In Chemistry When an acid and alkali react together, a neutralisation. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration is the slow. Two Uses Of Titration In Chemistry.
From www.microlit.us
Uses of Burettes in Pharmaceutical Industry Diagrams & Functions Two Uses Of Titration In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is an experiment through which the concentration of an unknown acid or alkali can. Two Uses Of Titration In Chemistry.
From giodaqksz.blob.core.windows.net
Acid Base Titration Guide at Mary Mcgee blog Two Uses Of Titration In Chemistry A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration. Two Uses Of Titration In Chemistry.
From giocaqlgs.blob.core.windows.net
Error During Titration at Elizabeth Mccranie blog Two Uses Of Titration In Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. It is commonly used in analytical chemistry to. Titration is the slow addition. Two Uses Of Titration In Chemistry.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Two Uses Of Titration In Chemistry When an acid and alkali react together, a neutralisation. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. Perform and interpret titration calculations. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated.. Two Uses Of Titration In Chemistry.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Two Uses Of Titration In Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. When an acid and alkali react together, a neutralisation. Titration is a widely used technique to measure. Two Uses Of Titration In Chemistry.
From giodaqksz.blob.core.windows.net
Acid Base Titration Guide at Mary Mcgee blog Two Uses Of Titration In Chemistry Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration is the slow addition of one solution of a known concentration. Two Uses Of Titration In Chemistry.
From boisestate.pressbooks.pub
14.7 AcidBase Titrations General Chemistry 1 & 2 Two Uses Of Titration In Chemistry When an acid and alkali react together, a neutralisation. It is commonly used in analytical chemistry to. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of. Two Uses Of Titration In Chemistry.
From mungfali.com
Acid Base Titration Calculation Two Uses Of Titration In Chemistry When an acid and alkali react together, a neutralisation. Perform and interpret titration calculations. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known. Two Uses Of Titration In Chemistry.
From www.pinterest.cl
chemistry of titration Gcse chemistry, Chemistry, Science chemistry Two Uses Of Titration In Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. When an acid and alkali react together, a neutralisation. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. Perform and interpret titration. Two Uses Of Titration In Chemistry.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Two Uses Of Titration In Chemistry When an acid and alkali react together, a neutralisation. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. It is commonly used in analytical chemistry to. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A. Two Uses Of Titration In Chemistry.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Two Uses Of Titration In Chemistry Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. When an acid and alkali react together, a neutralisation. A titration is. Two Uses Of Titration In Chemistry.
From augensternjiang.github.io
Chemistry Experiments and important reactions —— Common experiments Two Uses Of Titration In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is an experiment through which the concentration of an unknown acid or alkali can. Two Uses Of Titration In Chemistry.
From buoiholo.edu.vn
รายการ 105+ ภาพ Titrate ทางการแพทย์ ใหม่ที่สุด Two Uses Of Titration In Chemistry Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is an experiment through which the. Two Uses Of Titration In Chemistry.
From www.scribd.com
Titration PDF Titration Chemistry Two Uses Of Titration In Chemistry It is commonly used in analytical chemistry to. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of. Two Uses Of Titration In Chemistry.
From giogcqhar.blob.core.windows.net
Titration Definition Psychology at ster blog Two Uses Of Titration In Chemistry Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Titration is the slow addition of one solution of a known concentration (called a. Two Uses Of Titration In Chemistry.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Two Uses Of Titration In Chemistry Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. A titration is a laboratory technique used to precisely measure molar concentration of an. Two Uses Of Titration In Chemistry.
From studiousguy.com
Titration Uses in Real Life StudiousGuy Two Uses Of Titration In Chemistry A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Perform and interpret titration calculations. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory. Two Uses Of Titration In Chemistry.
From giogydsvx.blob.core.windows.net
Titration In Analytical Chemistry at Aida Battle blog Two Uses Of Titration In Chemistry It is commonly used in analytical chemistry to. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. When an acid and alkali react together, a neutralisation. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. Two Uses Of Titration In Chemistry.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Two Uses Of Titration In Chemistry When an acid and alkali react together, a neutralisation. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration is the slow addition of one solution of a known concentration (called. Two Uses Of Titration In Chemistry.
From quizizz.com
Titrations Chemistry Quiz Quizizz Two Uses Of Titration In Chemistry When an acid and alkali react together, a neutralisation. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is commonly used in analytical chemistry to. A titration is an experiment through which the concentration of an unknown acid or alkali can be calculated. Perform. Two Uses Of Titration In Chemistry.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Two Uses Of Titration In Chemistry It is commonly used in analytical chemistry to. Perform and interpret titration calculations. When an acid and alkali react together, a neutralisation. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known. Two Uses Of Titration In Chemistry.