Rate Constant K Chemistry . It is also known as the reaction rate. The rate constant (k) of a reaction can be calculated using: A rate constant, \(k\), is a. A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. The reaction orders in a rate law describe the. The composition of the equilibrium. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The ratio of the rate constants for the forward and reverse reactions at equilibrium is the equilibrium constant (\(k\)), a unitless quantity. Rate laws and rate constants. The initial rates and the rate equation;
from chem.libretexts.org
The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The ratio of the rate constants for the forward and reverse reactions at equilibrium is the equilibrium constant (\(k\)), a unitless quantity. It is also known as the reaction rate. Rate laws and rate constants. The initial rates and the rate equation; The reaction orders in a rate law describe the. A rate constant, \(k\), is a. A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The rate constant (k) of a reaction can be calculated using:
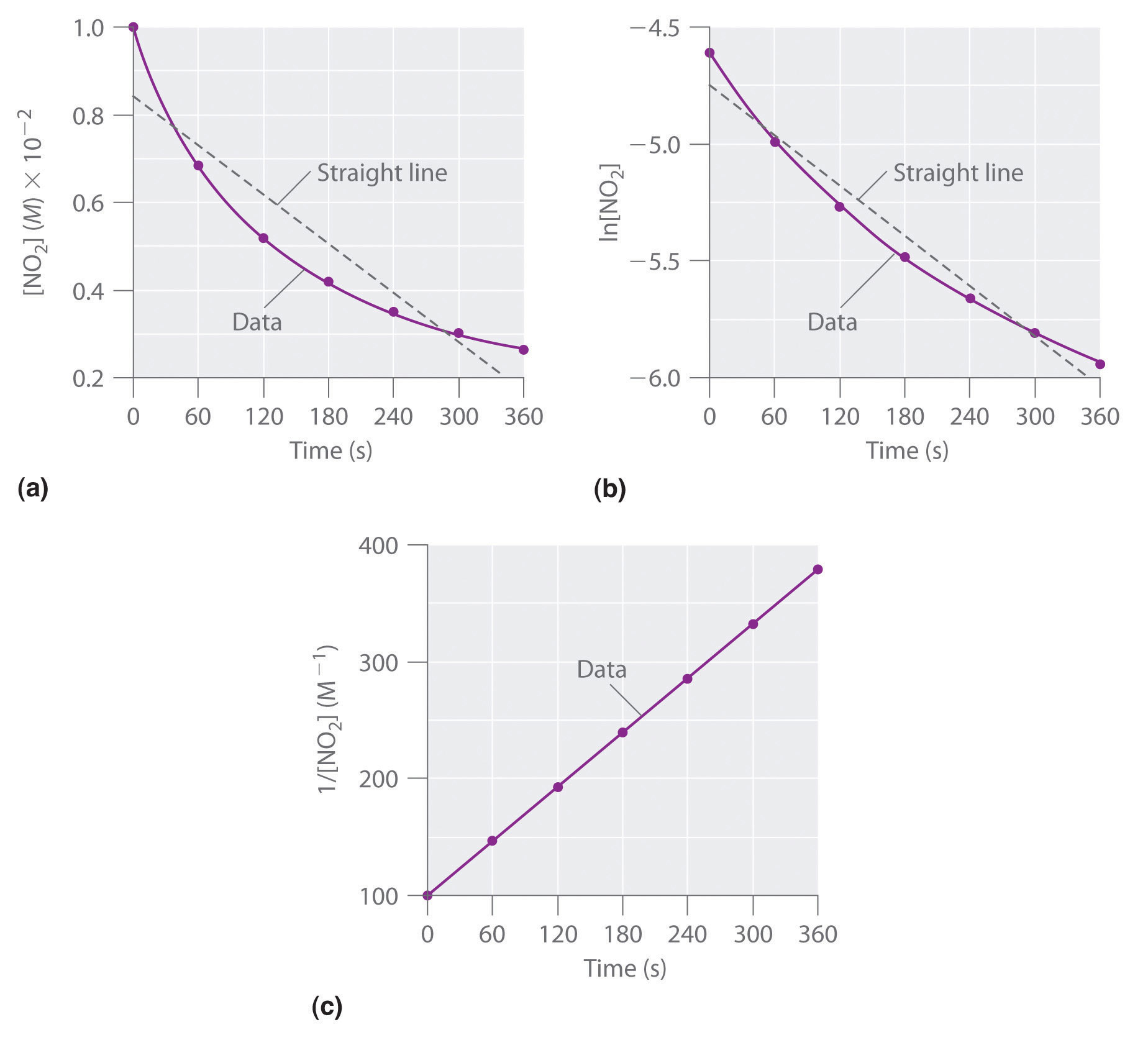
Chapter 13.4 Using Graphs to Determine Rate Laws, Rate Constants and
Rate Constant K Chemistry It is also known as the reaction rate. The ratio of the rate constants for the forward and reverse reactions at equilibrium is the equilibrium constant (\(k\)), a unitless quantity. A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. It is also known as the reaction rate. The composition of the equilibrium. Rate laws and rate constants. The reaction orders in a rate law describe the. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. A rate constant, \(k\), is a. The initial rates and the rate equation; The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The rate constant (k) of a reaction can be calculated using:
From www.sliderbase.com
Determining Order with Concentration vs. Time data Rate Constant K Chemistry The initial rates and the rate equation; The rate constant (k) of a reaction can be calculated using: Rate laws and rate constants. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of. Rate Constant K Chemistry.
From www.sliderbase.com
Determining Order with Concentration vs. Time data Rate Constant K Chemistry A rate constant, \(k\), is a. Rate laws and rate constants. The reaction orders in a rate law describe the. It is also known as the reaction rate. The ratio of the rate constants for the forward and reverse reactions at equilibrium is the equilibrium constant (\(k\)), a unitless quantity. The initial rates and the rate equation; The rate constant. Rate Constant K Chemistry.
From www.slideserve.com
PPT Chemistry 102(01) Spring 2012 PowerPoint Presentation, free Rate Constant K Chemistry The rate constant (k) of a reaction can be calculated using: The composition of the equilibrium. Rate laws and rate constants. The initial rates and the rate equation; It is also known as the reaction rate. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes. Rate Constant K Chemistry.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant K Chemistry It is also known as the reaction rate. Rate laws and rate constants. The initial rates and the rate equation; A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. The rate constant (k) of a reaction can be calculated using: The rate constant is a proportionality. Rate Constant K Chemistry.
From ashleeminnash.blogspot.com
Rate of Reaction Formula AshleeminNash Rate Constant K Chemistry The initial rates and the rate equation; The ratio of the rate constants for the forward and reverse reactions at equilibrium is the equilibrium constant (\(k\)), a unitless quantity. A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. It is also known as the reaction rate.. Rate Constant K Chemistry.
From chem.libretexts.org
Chapter 13.4 Using Graphs to Determine Rate Laws, Rate Constants and Rate Constant K Chemistry It is also known as the reaction rate. The initial rates and the rate equation; The rate constant (k) of a reaction can be calculated using: Rate laws and rate constants. A rate constant, \(k\), is a. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction. Rate Constant K Chemistry.
From www.tpsearchtool.com
How To Calculate The Order Of A Reaction And The Rate Constant Youtube Rate Constant K Chemistry Rate laws and rate constants. It is also known as the reaction rate. A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate.. Rate Constant K Chemistry.
From pametno21.blogspot.com
K Constant Formula Chemistry pametno Rate Constant K Chemistry The initial rates and the rate equation; Rate laws and rate constants. A rate constant, \(k\), is a. The reaction orders in a rate law describe the. A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. It is also known as the reaction rate. The rate. Rate Constant K Chemistry.
From study.com
Identifying HalfLife Given the Rate Constant Chemistry Rate Constant K Chemistry A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. A rate constant, \(k\), is a. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. Rate laws and rate constants.. Rate Constant K Chemistry.
From suhanaorlla.blogspot.com
9+ Calculating Rate Constant SuhanaOrlla Rate Constant K Chemistry The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. A rate law is an expression which relates that rate. Rate Constant K Chemistry.
From byjus.com
The rate constant of a reaction is 1.2×10^ 3sec^ 1 at 30℃and 2.1×10 Rate Constant K Chemistry A rate constant, \(k\), is a. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. Rate laws and rate constants. The initial rates and the rate equation; A rate law is an expression which relates that rate of a reaction to the rate. Rate Constant K Chemistry.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Rate Constant K Chemistry The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The ratio of the rate constants for the forward and reverse reactions at equilibrium is the equilibrium constant (\(k\)), a unitless quantity. The reaction orders in a rate law describe the. The rate constant is a proportionality factor in the rate law of chemical. Rate Constant K Chemistry.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical Rate Constant K Chemistry The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. The reaction orders in a rate law describe the. The composition of the equilibrium. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The rate constant (k). Rate Constant K Chemistry.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Rate Constant K Chemistry A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. It is also known as the reaction rate. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The initial rates and the. Rate Constant K Chemistry.
From facts.net
13 Mindblowing Facts About Reaction Rate Constant Rate Constant K Chemistry The composition of the equilibrium. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. A rate constant, \(k\), is a. The initial rates and the rate equation; The rate. Rate Constant K Chemistry.
From www.vrogue.co
K Value Units Chemistry vrogue.co Rate Constant K Chemistry The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. It is also known as the reaction rate. A rate constant, \(k\), is a. The composition of the equilibrium. The reaction orders in a rate law describe the. The rate constant is a proportionality. Rate Constant K Chemistry.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant K Chemistry Rate laws and rate constants. A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. It is also known as the reaction rate.. Rate Constant K Chemistry.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q Rate Constant K Chemistry The reaction orders in a rate law describe the. The rate constant (k) of a reaction can be calculated using: A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the. Rate Constant K Chemistry.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Rate Constant K Chemistry It is also known as the reaction rate. The initial rates and the rate equation; The ratio of the rate constants for the forward and reverse reactions at equilibrium is the equilibrium constant (\(k\)), a unitless quantity. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. Rate laws and rate constants. The rate. Rate Constant K Chemistry.
From ar.inspiredpencil.com
Rate Constant Rate Constant K Chemistry The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. The rate constant k and the exponents m, n, and p must be. Rate Constant K Chemistry.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free Rate Constant K Chemistry The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The ratio of the rate constants for the forward and reverse reactions at equilibrium is the equilibrium constant (\(k\)), a unitless quantity. A rate constant, \(k\), is a. A rate law is an expression which relates. Rate Constant K Chemistry.
From oneclass.com
OneClass The rate constant k for a certain reaction is measured at two Rate Constant K Chemistry The composition of the equilibrium. The rate constant (k) of a reaction can be calculated using: It is also known as the reaction rate. The reaction orders in a rate law describe the. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The rate constant is a proportionality factor in the rate law. Rate Constant K Chemistry.
From www.toppr.com
Rate constant k for first order reaction has been found to be 2.54 × 10 Rate Constant K Chemistry It is also known as the reaction rate. A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. The reaction orders in a rate law describe the. A rate constant, \(k\), is a. The composition of the equilibrium. The rate constant k is independent of the reactant. Rate Constant K Chemistry.
From www.slideserve.com
PPT SCH4U Unit 1 Energy Changes & Rates of Reactions (Cont’d Rate Constant K Chemistry The rate constant (k) of a reaction can be calculated using: The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. It is. Rate Constant K Chemistry.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant K Chemistry The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. The composition of the equilibrium. Rate laws and rate constants. It is also known as the reaction rate.. Rate Constant K Chemistry.
From www.sliderbase.com
Determining Order with Concentration vs. Time data Rate Constant K Chemistry Rate laws and rate constants. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. A rate law is an expression which relates that rate of a reaction to the. Rate Constant K Chemistry.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q Rate Constant K Chemistry The rate constant (k) of a reaction can be calculated using: A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. The initial rates and the rate equation; The composition of the equilibrium. The ratio of the rate constants for the forward and reverse reactions at equilibrium. Rate Constant K Chemistry.
From general.chemistrysteps.com
Equilibrium Constant Chemistry Steps Rate Constant K Chemistry A rate constant, \(k\), is a. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The reaction orders in a rate law describe the. The ratio of. Rate Constant K Chemistry.
From www.chemistrystudent.com
The Rate Equation (ALevel) ChemistryStudent Rate Constant K Chemistry The ratio of the rate constants for the forward and reverse reactions at equilibrium is the equilibrium constant (\(k\)), a unitless quantity. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The rate constant (k) of a reaction can be calculated using: The composition of the equilibrium. The reaction orders in a rate. Rate Constant K Chemistry.
From www.youtube.com
Rate equation and the units of the rate constant YouTube Rate Constant K Chemistry The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The rate constant (k) of a reaction can be calculated using: The reaction orders in a rate law describe the. The composition of the equilibrium. A rate constant, \(k\), is a. Rate laws and rate constants.. Rate Constant K Chemistry.
From www.slideshare.net
Chemical Rate Constant K Chemistry The composition of the equilibrium. A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the reactants. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates. Rate Constant K Chemistry.
From chemistryguru.com.sg
Rate Equation and Order of Reaction Rate Constant K Chemistry The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. The composition of the equilibrium. The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; The rate constant is a proportionality factor in the rate law. Rate Constant K Chemistry.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube Rate Constant K Chemistry The rate constant (k) of a reaction can be calculated using: The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. The reaction orders in a rate law describe the. A rate constant, \(k\), is a. The composition of the equilibrium. A rate law. Rate Constant K Chemistry.
From www.youtube.com
Chemical Initial Rates Method YouTube Rate Constant K Chemistry The rate constant (k) of a reaction can be calculated using: Rate laws and rate constants. The ratio of the rate constants for the forward and reverse reactions at equilibrium is the equilibrium constant (\(k\)), a unitless quantity. The composition of the equilibrium. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. A. Rate Constant K Chemistry.
From www.chegg.com
Solved Chemical , Rate Law, k Using experimented Rate Constant K Chemistry The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a reaction changes as the. The initial rates and the rate equation; The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. It is also. Rate Constant K Chemistry.