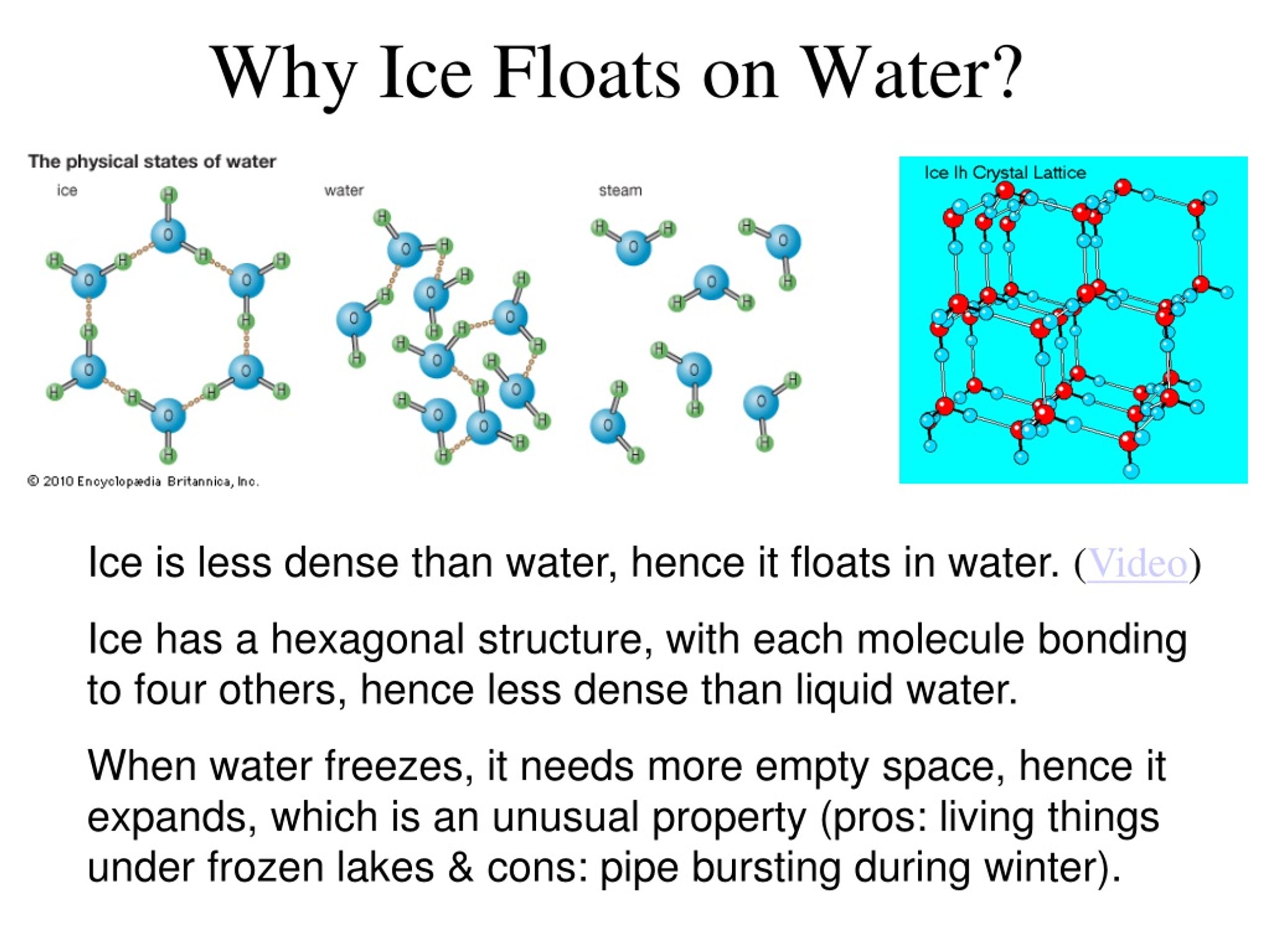
Ice Floats Def . Ice cubes float because of their molecular structure. The hydrogen and oxygen atoms share electron pairs, forming. For something to float, the upward buoyant force must be at least. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. For an object to float in water, its buoyant force has to be at least as big as its weight. However, this is a peculiar behavior as solid matter. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. Ice floats because it is about 9% less dense than liquid water. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Discover the intriguing phenomenon of ice defying intuition and floating in water, attributed to its lower density resulting from distinctive. Understanding why ice floats is essential not. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. When you put the ice into the water, the denser water pushes the ice to the top where it will float. The heavier water displaces the lighter ice, so ice floats to the top. Because ice floats, bodies of water freeze from top to bottom.
from www.slideserve.com
The heavier water displaces the lighter ice, so ice floats to the top. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Ice cubes float because of their molecular structure. However, this is a peculiar behavior as solid matter. The hydrogen and oxygen atoms share electron pairs, forming. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. Understanding why ice floats is essential not. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans.
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download
Ice Floats Def The heavier water displaces the lighter ice, so ice floats to the top. For something to float, the upward buoyant force must be at least. The hydrogen and oxygen atoms share electron pairs, forming. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. However, this is a peculiar behavior as solid matter. For an object to float in water, its buoyant force has to be at least as big as its weight. Because ice floats, bodies of water freeze from top to bottom. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. The heavier water displaces the lighter ice, so ice floats to the top. Ice floats because it is about 9% less dense than liquid water. When you put the ice into the water, the denser water pushes the ice to the top where it will float. Understanding why ice floats is essential not. Discover the intriguing phenomenon of ice defying intuition and floating in water, attributed to its lower density resulting from distinctive. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation, free download ID5431394 Ice Floats Def Ice cubes float because of their molecular structure. Ice floats because it is about 9% less dense than liquid water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. Because ice floats, bodies. Ice Floats Def.
From www.slideserve.com
PPT WATER REVIEW PowerPoint Presentation, free download ID2220597 Ice Floats Def The heavier water displaces the lighter ice, so ice floats to the top. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. Ice floats because it is about 9% less dense than liquid water. For something to float, the upward buoyant force must be at least. Discover the. Ice Floats Def.
From www.slideserve.com
PPT Energy and the States Of Matter PowerPoint Presentation, free Ice Floats Def Understanding why ice floats is essential not. Ice floats because it is about 9% less dense than liquid water. Because ice floats, bodies of water freeze from top to bottom. Ice cubes float because of their molecular structure. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. For something to float, the upward buoyant force. Ice Floats Def.
From www.slideserve.com
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download Ice Floats Def The hydrogen and oxygen atoms share electron pairs, forming. For an object to float in water, its buoyant force has to be at least as big as its weight. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. For something to float, the upward buoyant force must. Ice Floats Def.
From dissolve.com
Ice floats floating in the sea, South Island, South Sandwich Ice Floats Def For an object to float in water, its buoyant force has to be at least as big as its weight. Because ice floats, bodies of water freeze from top to bottom. Ice floats because it is about 9% less dense than liquid water. Understanding why ice floats is essential not. When you put the ice into the water, the denser. Ice Floats Def.
From www.slideserve.com
PPT Chapter 3. Water— The Elixir of Life! PowerPoint Presentation Ice Floats Def It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. For something to float, the upward buoyant force must be at least. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water.. Ice Floats Def.
From www.slideserve.com
PPT L14 Fluids [3] PowerPoint Presentation, free download ID6186946 Ice Floats Def The hydrogen and oxygen atoms share electron pairs, forming. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Discover the intriguing phenomenon of ice defying intuition and floating in water, attributed to its lower density resulting from distinctive. When you put the ice into the water, the denser water pushes the ice to the top. Ice Floats Def.
From www.dreamstime.com
Melting Ice Floats on a River Water in Winter Stock Image Image of Ice Floats Def Discover the intriguing phenomenon of ice defying intuition and floating in water, attributed to its lower density resulting from distinctive. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. Ice floats because it is about 9% less dense than liquid water. The hydrogen and oxygen atoms share. Ice Floats Def.
From www.slideserve.com
PPT 2.1 Chemical Elements PowerPoint Presentation, free download ID Ice Floats Def However, this is a peculiar behavior as solid matter. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. Ice floats because it is about 9% less dense than liquid water. Ice cubes float. Ice Floats Def.
From www.slideserve.com
PPT The Chemistry of Life PowerPoint Presentation, free download ID Ice Floats Def A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Ice cubes float because of their molecular structure. When you put the ice into the water, the denser water pushes the ice to the top where it will float. This unique property of water is especially beneficial for fish that live in bodies of water that. Ice Floats Def.
From slideplayer.com
Water Unit. ppt download Ice Floats Def Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. Ice floats because it is about 9% less dense than liquid water. Ice cubes float because of their molecular structure. In other words, ice takes up about 9% more space than water, so a liter of ice weighs. Ice Floats Def.
From www.slideserve.com
PPT Water, Water, Everywhere (Ch. 3) PowerPoint Presentation, free Ice Floats Def Discover the intriguing phenomenon of ice defying intuition and floating in water, attributed to its lower density resulting from distinctive. For something to float, the upward buoyant force must be at least. Because ice floats, bodies of water freeze from top to bottom. Ice cubes float because of their molecular structure. A water molecule (h2o) is made of two hydrogen. Ice Floats Def.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download Ice Floats Def For an object to float in water, its buoyant force has to be at least as big as its weight. Because ice floats, bodies of water freeze from top to bottom. When you put the ice into the water, the denser water pushes the ice to the top where it will float. Ice floats because it is about 9% less. Ice Floats Def.
From www.youtube.com
Why does ice float on water? Detailed Explanation YouTube Ice Floats Def In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Ice cubes float because of their molecular structure. However, this is a peculiar behavior as solid matter. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter.. Ice Floats Def.
From www.interestingfacts.org
Why Does Ice Float On Water Definition In Alcohol Ice Floats Def Understanding why ice floats is essential not. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. The hydrogen and oxygen atoms share electron pairs, forming. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Because ice floats, bodies of water freeze from top. Ice Floats Def.
From slideplayer.com
Unit 1 The Chemistry of Life ppt download Ice Floats Def For an object to float in water, its buoyant force has to be at least as big as its weight. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. When you put the ice into the water, the denser water pushes the ice to the top where. Ice Floats Def.
From www.youtube.com
Why ice floats on liquid water? Why volume of water increases on Ice Floats Def Discover the intriguing phenomenon of ice defying intuition and floating in water, attributed to its lower density resulting from distinctive. Ice cubes float because of their molecular structure. However, this is a peculiar behavior as solid matter. Understanding why ice floats is essential not. It is common for us to observe ice cubes floating when placed in a glass of. Ice Floats Def.
From www.thoughtco.com
Ice and the Density of Water Ice Floats Def This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. For something to float, the upward buoyant force must be at least. Ice cubes float because of their molecular structure. Because ice floats, bodies of water freeze from top to bottom. A water molecule (h2o) is made of two. Ice Floats Def.
From www.slideserve.com
PPT Chapter 17 Water and Aqueous Systems PowerPoint Presentation Ice Floats Def However, this is a peculiar behavior as solid matter. Because ice floats, bodies of water freeze from top to bottom. The hydrogen and oxygen atoms share electron pairs, forming. Ice cubes float because of their molecular structure. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. Ice Floats Def.
From www.slideserve.com
PPT The Chemistry of Life PowerPoint Presentation, free download ID Ice Floats Def The hydrogen and oxygen atoms share electron pairs, forming. Ice cubes float because of their molecular structure. Discover the intriguing phenomenon of ice defying intuition and floating in water, attributed to its lower density resulting from distinctive. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. For an. Ice Floats Def.
From www.slideserve.com
PPT How’s your grade? PowerPoint Presentation, free download ID2873318 Ice Floats Def For an object to float in water, its buoyant force has to be at least as big as its weight. The hydrogen and oxygen atoms share electron pairs, forming. Because ice floats, bodies of water freeze from top to bottom. Understanding why ice floats is essential not. Unlike most substances, water behaves in an unusual way when it freezes, leading. Ice Floats Def.
From www.worldatlas.com
Why Does Ice Float? Ice Floats Def The hydrogen and oxygen atoms share electron pairs, forming. For an object to float in water, its buoyant force has to be at least as big as its weight. Ice floats because it is about 9% less dense than liquid water. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density. Ice Floats Def.
From thevigyan.com
Why Does Ice Float in Water? The Vigyan Ice Floats Def The heavier water displaces the lighter ice, so ice floats to the top. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Ice cubes float because of their molecular structure. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and. Ice Floats Def.
From www.needpix.com
Water,ice,float,floating,melt free image from Ice Floats Def A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Discover the intriguing phenomenon of ice defying intuition and floating in water, attributed to its lower density resulting from distinctive. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. For an object to. Ice Floats Def.
From www.youtube.com
Why Ice Floats on Water YouTube Ice Floats Def A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. However, this is a peculiar behavior as solid matter. Ice cubes float because of their molecular structure. The heavier water displaces the lighter ice, so ice floats to the top. For something to float, the upward buoyant force must be at least. Understanding why ice floats. Ice Floats Def.
From www.sciencefriday.com
Use Flubber to Model Ice ShelfGlacier Interactions and Sea Level Rise Ice Floats Def However, this is a peculiar behavior as solid matter. For an object to float in water, its buoyant force has to be at least as big as its weight. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. Discover the intriguing phenomenon of ice defying intuition and floating. Ice Floats Def.
From www.slideserve.com
PPT Oceanography PowerPoint Presentation ID4248517 Ice Floats Def Because ice floats, bodies of water freeze from top to bottom. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. For something to float, the upward buoyant force must be at least. Understanding why ice floats is essential not. Ice cubes float because. Ice Floats Def.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download Ice Floats Def Discover the intriguing phenomenon of ice defying intuition and floating in water, attributed to its lower density resulting from distinctive. When you put the ice into the water, the denser water pushes the ice to the top where it will float. Understanding why ice floats is essential not. It is common for us to observe ice cubes floating when placed. Ice Floats Def.
From www.scribd.com
Ice Floats On Water PDF Ice Floats Def In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. For something to float, the upward buoyant force must be at least. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. The hydrogen and oxygen atoms share electron pairs, forming. Unlike most substances,. Ice Floats Def.
From www.slideserve.com
PPT Characteristics of Water PowerPoint Presentation ID3103706 Ice Floats Def In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. However, this is a peculiar behavior as solid matter. The heavier water displaces the lighter ice, so. Ice Floats Def.
From www.slideserve.com
PPT Chapter 3 Chemical and Physical Features of Seawater PowerPoint Ice Floats Def Ice floats because it is about 9% less dense than liquid water. The hydrogen and oxygen atoms share electron pairs, forming. When you put the ice into the water, the denser water pushes the ice to the top where it will float. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower. Ice Floats Def.
From www.slideserve.com
PPT Ch 1 The Chemistry of Life PowerPoint Presentation, free Ice Floats Def Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. Understanding why ice floats is essential not. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. A water molecule (h2o) is made of two hydrogen atoms. Ice Floats Def.
From www.youtube.com
Ice Floats in Water Why does Ice Float? FACTS 23 YouTube Ice Floats Def Ice cubes float because of their molecular structure. When you put the ice into the water, the denser water pushes the ice to the top where it will float. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. It is common for us to observe ice cubes floating. Ice Floats Def.
From www.slideserve.com
PPT Intermolecular Force PowerPoint Presentation, free download ID Ice Floats Def Because ice floats, bodies of water freeze from top to bottom. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. Discover the intriguing phenomenon of ice. Ice Floats Def.
From www.interestingfacts.org
Why Does Ice Float On Water Definition In Alcohol Ice Floats Def Ice cubes float because of their molecular structure. This unique property of water is especially beneficial for fish that live in bodies of water that freeze in the winter. Understanding why ice floats is essential not. Discover the intriguing phenomenon of ice defying intuition and floating in water, attributed to its lower density resulting from distinctive. However, this is a. Ice Floats Def.