Titration Curve Between Acetic Acid And Naoh . The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly with naoh. In this case, there is a very sharp transition in. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Titration curve (acetic acid and naoh). Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. The shape of the curve provides important information about what is occurring in. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. The titration curve is shown below. The equivalence point of a titration. Sorting out some confusing terms. A titration involves performing a controlled reaction.
from www.chegg.com
The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. The shape of the curve provides important information about what is occurring in. The titration curve is shown below. The equivalence point of a titration. A titration involves performing a controlled reaction. In this case, there is a very sharp transition in. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Sorting out some confusing terms. Titration curve (acetic acid and naoh).
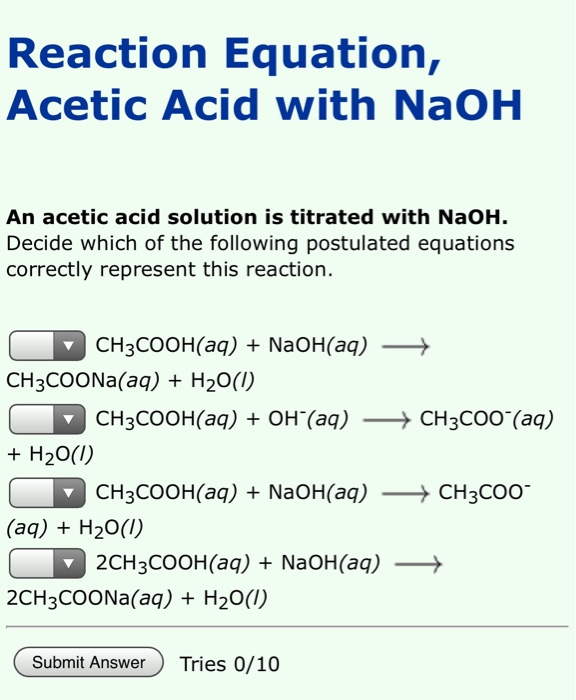
Solved Reaction Equation, Acetic Acid with NaOH An acetic
Titration Curve Between Acetic Acid And Naoh The shape of the curve provides important information about what is occurring in. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. A titration involves performing a controlled reaction. Sorting out some confusing terms. The titration curve is shown below. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. In this case, there is a very sharp transition in. The equivalence point of a titration. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. Titration curve (acetic acid and naoh). The shape of the curve provides important information about what is occurring in. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly with naoh.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Titration Curve Between Acetic Acid And Naoh The titration curve is shown below. The equivalence point of a titration. In this case, there is a very sharp transition in. The shape of the curve provides important information about what is occurring in. Sorting out some confusing terms. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid. Titration Curve Between Acetic Acid And Naoh.
From wizedu.com
3. Plot a schematic titration curve for acetic acid with NaOH Titration Curve Between Acetic Acid And Naoh The shape of the curve provides important information about what is occurring in. Titration curve (acetic acid and naoh). In this case, there is a very sharp transition in. The equivalence point of a titration. The titration curve is shown below. A titration involves performing a controlled reaction. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting. Titration Curve Between Acetic Acid And Naoh.
From dxomwdfni.blob.core.windows.net
Titration Ph Formula at Farrah Carleton blog Titration Curve Between Acetic Acid And Naoh In this case, there is a very sharp transition in. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. When titrating a strong acid, such as hydrochloric. Titration Curve Between Acetic Acid And Naoh.
From www.chegg.com
Solved Reaction Equation, Acetic Acid with NaOH An acetic Titration Curve Between Acetic Acid And Naoh In this case, there is a very sharp transition in. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Sorting out some confusing terms. Titration curve (acetic acid and naoh). A titration involves performing a controlled reaction. The equivalence point of a titration. The titration. Titration Curve Between Acetic Acid And Naoh.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Titration Curve Between Acetic Acid And Naoh In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Titration curve (acetic acid and naoh). The equivalence point of a titration. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly with naoh. Sorting out some confusing terms. In this. Titration Curve Between Acetic Acid And Naoh.
From www.numerade.com
SOLVED 0 + 5 Acetic acid titrated with NaOH 10 pH Volume of NaOH (mL Titration Curve Between Acetic Acid And Naoh A titration involves performing a controlled reaction. In this case, there is a very sharp transition in. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The equivalence point of a titration. Plotting the ph of the solution in the flask against the amount of. Titration Curve Between Acetic Acid And Naoh.
From mungfali.com
Titration Curve Of Acetic Acid Titration Curve Between Acetic Acid And Naoh Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. In this case, there is a very sharp transition in. The equivalence point of a titration. The titration. Titration Curve Between Acetic Acid And Naoh.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Titration Curve Between Acetic Acid And Naoh The titration curve is shown below. The equivalence point of a titration. A titration involves performing a controlled reaction. Titration curve (acetic acid and naoh). In this case, there is a very sharp transition in. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Sorting out some confusing. Titration Curve Between Acetic Acid And Naoh.
From mavink.com
Titration Curve Of Acetic Acid Titration Curve Between Acetic Acid And Naoh In this case, there is a very sharp transition in. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly with naoh. The titration curve is shown below. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. Sorting. Titration Curve Between Acetic Acid And Naoh.
From www.slideshare.net
Water And Buffers Titration Curve Between Acetic Acid And Naoh In this case, there is a very sharp transition in. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The shape of the curve provides important information about what is occurring in. Titration curve (acetic acid and naoh). A titration involves performing a controlled reaction.. Titration Curve Between Acetic Acid And Naoh.
From mavink.com
Acetic Acid And Naoh Titration Titration Curve Between Acetic Acid And Naoh When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly with naoh. The equivalence point of a titration. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The titration curve is shown below. Titration curve (acetic acid. Titration Curve Between Acetic Acid And Naoh.
From www.researchgate.net
Titration curve of monobasic acid (0.1 M acetic acid). Download Titration Curve Between Acetic Acid And Naoh The titration curve is shown below. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly with naoh. In this case, there is a very sharp transition in. A titration involves performing a controlled reaction. In this experiment, a technique known as a titration will be used to determine the concentration of. Titration Curve Between Acetic Acid And Naoh.
From mungfali.com
Endpoint Titration Curve Titration Curve Between Acetic Acid And Naoh The shape of the curve provides important information about what is occurring in. In this case, there is a very sharp transition in. Sorting out some confusing terms. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. When titrating a strong acid, such as hydrochloric acid with sodium. Titration Curve Between Acetic Acid And Naoh.
From www.numerade.com
SOLVEDHel AXue Ekpad Scraenshot HAdAm; Heueplal Buffer titration curve Titration Curve Between Acetic Acid And Naoh A titration involves performing a controlled reaction. The equivalence point of a titration. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. When titrating a. Titration Curve Between Acetic Acid And Naoh.
From www.researchgate.net
Titration curves for acetic acid, monochloroacetic acid and Titration Curve Between Acetic Acid And Naoh Sorting out some confusing terms. Titration curve (acetic acid and naoh). The equivalence point of a titration. A titration involves performing a controlled reaction. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly with naoh. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic. Titration Curve Between Acetic Acid And Naoh.
From www.writework.com
Titration of amino acids WriteWork Titration Curve Between Acetic Acid And Naoh Sorting out some confusing terms. In this case, there is a very sharp transition in. The shape of the curve provides important information about what is occurring in. Titration curve (acetic acid and naoh). Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. The equivalence point of a. Titration Curve Between Acetic Acid And Naoh.
From mavink.com
Titration Of Acetic Acid With Naoh Titration Curve Between Acetic Acid And Naoh The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. A titration involves performing a controlled reaction. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. Titration curve (acetic acid and naoh).. Titration Curve Between Acetic Acid And Naoh.
From mavink.com
Titration Curve Of Acetic Acid Titration Curve Between Acetic Acid And Naoh A titration involves performing a controlled reaction. The equivalence point of a titration. The shape of the curve provides important information about what is occurring in. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. The titration curve is shown below. The titration curve in figure \(\pageindex{3a}\) was. Titration Curve Between Acetic Acid And Naoh.
From www.chegg.com
Solved Use the following titration curve for the first four Titration Curve Between Acetic Acid And Naoh The equivalence point of a titration. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. In this case, there is a very sharp transition in. Sorting out some confusing terms. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution. Titration Curve Between Acetic Acid And Naoh.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Titration Curve Between Acetic Acid And Naoh In this case, there is a very sharp transition in. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. The titration curve is shown below. Sorting out some confusing terms. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the. Titration Curve Between Acetic Acid And Naoh.
From goodttorials.blogspot.com
How To Find Equivalence Point From Titration Data Titration Curve Between Acetic Acid And Naoh The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The shape of the curve provides important information about what is occurring in. Sorting out some confusing terms. In this case, there is a very sharp transition in. Plotting the ph of the solution in the. Titration Curve Between Acetic Acid And Naoh.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Titration Curve Between Acetic Acid And Naoh The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Plotting the ph of the solution in the flask against the amount of. Titration Curve Between Acetic Acid And Naoh.
From exogrnoza.blob.core.windows.net
Titration Curve Acetic Acid And Naoh at Isabel Keith blog Titration Curve Between Acetic Acid And Naoh The shape of the curve provides important information about what is occurring in. The equivalence point of a titration. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. Titration curve (acetic acid and naoh). The titration of a weak acid with a strong base involves the. Titration Curve Between Acetic Acid And Naoh.
From byjus.com
titration is carried out in between N/10 acetic acid and N/10 NaOH .how Titration Curve Between Acetic Acid And Naoh The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. A titration involves performing a controlled reaction. The shape of the curve provides important information about what is occurring in. Sorting out some confusing terms. In this experiment, a technique known as a titration will be used. Titration Curve Between Acetic Acid And Naoh.
From childhealthpolicy.vumc.org
⭐ Acetic acid and naoh titration. Titration of NaOH with acetic acid Titration Curve Between Acetic Acid And Naoh The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. Titration curve (acetic acid and naoh). The equivalence point of a titration. In this case, there. Titration Curve Between Acetic Acid And Naoh.
From www.youtube.com
MCAT Acid/Base Equilibria Titration Curve of Weak Acid Strong Base Titration Curve Between Acetic Acid And Naoh The shape of the curve provides important information about what is occurring in. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly with naoh. Sorting out some confusing. Titration Curve Between Acetic Acid And Naoh.
From www.youtube.com
Titration Acetic Acid with NaOH YouTube Titration Curve Between Acetic Acid And Naoh In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. The shape of the curve provides important information about what is occurring in. The equivalence point of a titration. Titration curve (acetic acid and naoh). Sorting out some confusing terms. Plotting the ph of the solution in the flask. Titration Curve Between Acetic Acid And Naoh.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Titration Curve Between Acetic Acid And Naoh The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you. Titration Curve Between Acetic Acid And Naoh.
From mungfali.com
Titration Curve Of Acetic Acid Titration Curve Between Acetic Acid And Naoh The titration curve is shown below. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. In this case, there is a very sharp transition in. Titration curve (acetic acid and naoh). The shape of the curve provides important information about what is occurring in. The titration of a. Titration Curve Between Acetic Acid And Naoh.
From www.numerade.com
SOLVED Titration curve for the Titration of of 25.0 mL of Acetic Acid Titration Curve Between Acetic Acid And Naoh When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly with naoh. In this case, there is a very sharp transition in. In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Titration curve (acetic acid and naoh). The titration curve. Titration Curve Between Acetic Acid And Naoh.
From www.researchgate.net
Titration curve of acetic acid at 30 °C (Ve = equivalence volume Titration Curve Between Acetic Acid And Naoh A titration involves performing a controlled reaction. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. Titration curve (acetic acid and naoh). Sorting out some confusing terms. When titrating a strong acid, such as hydrochloric acid with sodium hydroxide, you are reacting the hcl directly with naoh. The. Titration Curve Between Acetic Acid And Naoh.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Between Acetic Acid And Naoh The shape of the curve provides important information about what is occurring in. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Titration curve (acetic acid and naoh). The titration curve is shown below. Plotting the ph of the solution in the flask against the. Titration Curve Between Acetic Acid And Naoh.
From feqtudy.blogspot.com
Indicator For Titration Of Acetic Acid And Sodium Hydroxide FEQTUDY Titration Curve Between Acetic Acid And Naoh The titration curve is shown below. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. Sorting out some confusing terms. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. A titration. Titration Curve Between Acetic Acid And Naoh.
From www.researchgate.net
Titration of acetic acid with sodium hydroxide. 1) calculated titration Titration Curve Between Acetic Acid And Naoh The shape of the curve provides important information about what is occurring in. The titration curve in figure \(\pageindex{3a}\) was created by calculating the starting ph of the acetic acid solution before any \(\ce{naoh}\) is added. The equivalence point of a titration. Plotting the ph of the solution in the flask against the amount of acid or base added produces. Titration Curve Between Acetic Acid And Naoh.
From www.youtube.com
Acetic Acid NaOH Titration Curve YouTube Titration Curve Between Acetic Acid And Naoh The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. A titration involves performing a controlled reaction. Titration curve (acetic acid and naoh). Sorting out some confusing terms. The titration curve is shown below. The equivalence point of a titration. The titration curve in figure \(\pageindex{3a}\). Titration Curve Between Acetic Acid And Naoh.