Chlorine + Excess Ammonia . Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on the number of reactants taken for the reaction. Naocl + 2hcl → cl 2 +. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. The reaction between ammonia and chlorine is much faster than the rate that chlorine kills bacteria so you cannot use chlorine to disinfect water that contains ammonia. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. Bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. Reaction of excess chlorine with less ammonia. The residual leaving the plant is 2.5. A water system is having trouble maintaining a residual at the end of the distribution system.
from www.doubtnut.com
Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. The residual leaving the plant is 2.5. A water system is having trouble maintaining a residual at the end of the distribution system. Naocl + 2hcl → cl 2 +. Bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on the number of reactants taken for the reaction. The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. The reaction between ammonia and chlorine is much faster than the rate that chlorine kills bacteria so you cannot use chlorine to disinfect water that contains ammonia. Reaction of excess chlorine with less ammonia. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts.
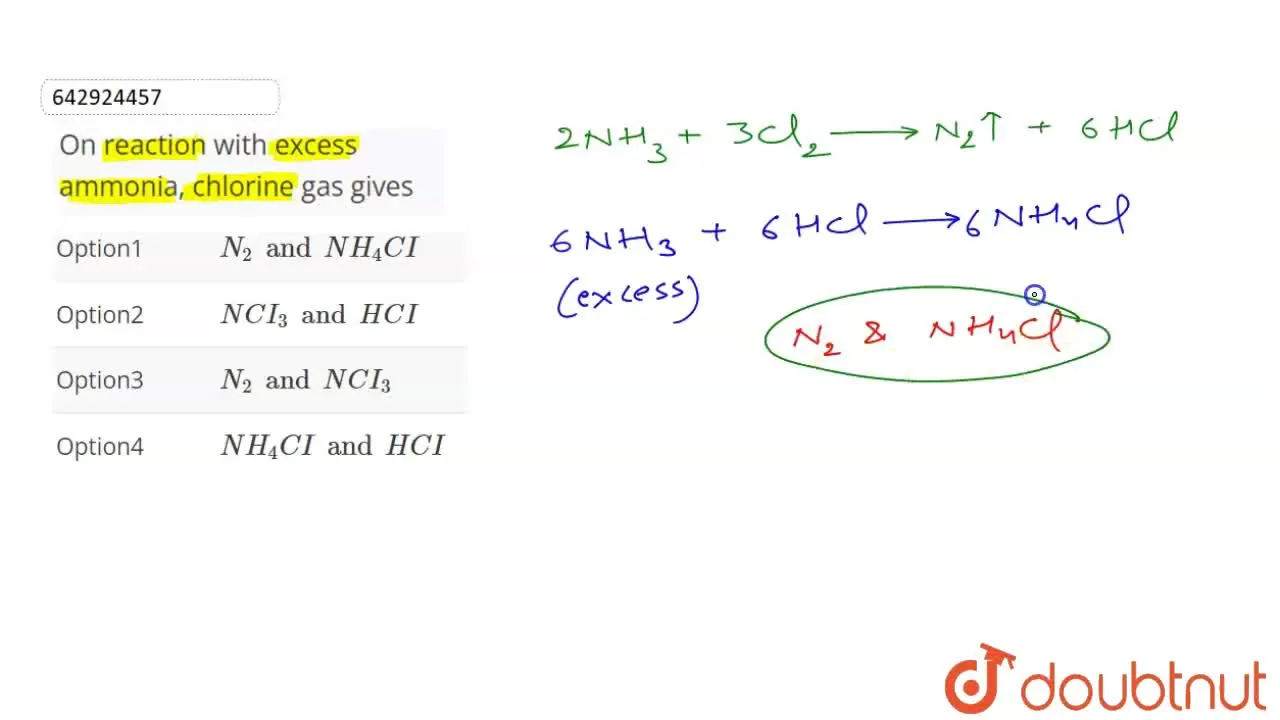
On reaction with excess ammonia, chlorine gas gives
Chlorine + Excess Ammonia Naocl + 2hcl → cl 2 +. When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. Naocl + 2hcl → cl 2 +. Bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. A water system is having trouble maintaining a residual at the end of the distribution system. Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on the number of reactants taken for the reaction. The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. The residual leaving the plant is 2.5. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. Reaction of excess chlorine with less ammonia. The reaction between ammonia and chlorine is much faster than the rate that chlorine kills bacteria so you cannot use chlorine to disinfect water that contains ammonia.
From www.numerade.com
SOLVEDWhen excess of ammonia and chlorine react, nitrogen and ammonium Chlorine + Excess Ammonia Naocl + 2hcl → cl 2 +. A water system is having trouble maintaining a residual at the end of the distribution system. The reaction between ammonia and chlorine is much faster than the rate that chlorine kills bacteria so you cannot use chlorine to disinfect water that contains ammonia. Chlorine (cl 2) gas reacts with ammonia (nh 3) gas. Chlorine + Excess Ammonia.
From www.nagwa.com
Question Video Selecting the Correction Equation for the Reversible Chlorine + Excess Ammonia The residual leaving the plant is 2.5. Reaction of excess chlorine with less ammonia. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour. Chlorine + Excess Ammonia.
From www.toppr.com
Chlorine reacts with excess of ammonia to form Chlorine + Excess Ammonia When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. The residual leaving the plant is 2.5. Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on the number of reactants taken for the reaction. The reaction between ammonia and chlorine is much faster than the. Chlorine + Excess Ammonia.
From cookinglove.com
Chlorine and ammonia Chlorine + Excess Ammonia Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. A water system is having trouble maintaining a residual at the end of the distribution system. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. The reaction between ammonia and chlorine is much faster than the rate. Chlorine + Excess Ammonia.
From askfilo.com
Chlorine is allowed to react with excess of ammonia. In this, 1 mole of c.. Chlorine + Excess Ammonia Bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. The reaction between ammonia and chlorine is much faster than the rate that chlorine kills bacteria so you cannot use chlorine to disinfect water that contains ammonia. The residual leaving. Chlorine + Excess Ammonia.
From zavierfersprince.blogspot.com
Ammonia Nh3 Reacts With Hydrogen Chloride to Form Ammonium Chloride Chlorine + Excess Ammonia When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. Reaction of excess chlorine with less ammonia. A water system is having trouble maintaining a residual at the end of the distribution system. The reaction between ammonia and chlorine is much faster than the rate that chlorine kills bacteria so you. Chlorine + Excess Ammonia.
From askfilo.com
(a) ammonia reacts with excess of chlorine? Filo Chlorine + Excess Ammonia When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. The residual leaving the plant is 2.5. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. Reaction of excess chlorine with less ammonia. Bleach reacts with hydrochloric acid to produce chlorine gas,. Chlorine + Excess Ammonia.
From askfilo.com
14 Treatment of ammonia with excess ethyl chloride will yield Filo Chlorine + Excess Ammonia When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. Naocl + 2hcl → cl 2 +. The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along. Chlorine + Excess Ammonia.
From www.youtube.com
On reaction with excess ammonia, chlorine gas gives nitrogen gas along Chlorine + Excess Ammonia The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. Bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. When an operator has trouble maintaining constant combined residuals in plant processes or in the. Chlorine + Excess Ammonia.
From www.doubtnut.com
On reaction with excess ammonia, chlorine gas gives Chlorine + Excess Ammonia Bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. Reaction of excess chlorine with less ammonia. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. Naocl + 2hcl → cl 2 +. The reaction. Chlorine + Excess Ammonia.
From www.youtube.com
Ammonia, on reaction with excess of chlorine, gives CLASS 12 THE P Chlorine + Excess Ammonia The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. A water system is having trouble maintaining a residual at the end of the distribution system. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen. Chlorine + Excess Ammonia.
From www.numerade.com
SOLVEDAmmonia rapidly reacts with hydrogen chloride, making ammonium Chlorine + Excess Ammonia Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. The residual leaving the plant is 2.5. Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on the number of reactants taken for the reaction. A water system is having trouble maintaining a residual at the end of the distribution system.. Chlorine + Excess Ammonia.
From www.doubtnut.com
Ammonia reacts with excess of chlorine to form Chlorine + Excess Ammonia Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. A water system is having trouble maintaining a residual at the end of the distribution system. The reaction between ammonia and chlorine is much faster than the. Chlorine + Excess Ammonia.
From www.numerade.com
SOLVED Ammonia rapidly reacts with hydrogen chloride, making ammonium Chlorine + Excess Ammonia Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. Naocl + 2hcl → cl 2 +. The reaction between ammonia and chlorine is much faster than the rate that chlorine kills bacteria. Chlorine + Excess Ammonia.
From cookinglove.com
Chlorine and ammonia Chlorine + Excess Ammonia A water system is having trouble maintaining a residual at the end of the distribution system. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. Naocl + 2hcl → cl 2 +. Chlorine (cl 2) gas reacts. Chlorine + Excess Ammonia.
From askfilo.com
Chlorine is allowed to react with excess of ammonia. In this, 1 mole of c.. Chlorine + Excess Ammonia A water system is having trouble maintaining a residual at the end of the distribution system. Naocl + 2hcl → cl 2 +. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. The reaction between ammonia and chlorine is much faster than the rate that chlorine kills bacteria so you cannot use chlorine to disinfect. Chlorine + Excess Ammonia.
From www.youtube.com
chlorine reacts with excess of ammonia to form YouTube Chlorine + Excess Ammonia The reaction between ammonia and chlorine is much faster than the rate that chlorine kills bacteria so you cannot use chlorine to disinfect water that contains ammonia. Naocl + 2hcl → cl 2 +. The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. Ammonia reacts with chlorine to make chloramine. Chlorine + Excess Ammonia.
From askfilo.com
Chlorine reacts with excess of ammonia to form [2021A] Filo Chlorine + Excess Ammonia Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on the number of reactants taken for the reaction. When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. Naocl + 2hcl → cl 2 +. Reaction of excess chlorine with less ammonia. Bleach reacts with hydrochloric. Chlorine + Excess Ammonia.
From www.toppr.com
When excess of ammonia and chlorine react, nitrogen and ammonium Chlorine + Excess Ammonia A water system is having trouble maintaining a residual at the end of the distribution system. The residual leaving the plant is 2.5. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. The reaction between ammonia and. Chlorine + Excess Ammonia.
From www.doubtnut.com
[Kannada] How excess of chlorine reacts with ammonia. Chlorine + Excess Ammonia The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. The residual leaving the plant is 2.5. Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on the number of reactants taken for the reaction. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen. Chlorine + Excess Ammonia.
From www.researchgate.net
Chlorine vs ammonia, 1 st set Download HighResolution Scientific Diagram Chlorine + Excess Ammonia When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. Reaction of excess chlorine with less ammonia. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. Nitrogen. Chlorine + Excess Ammonia.
From www.numerade.com
SOLVEDExplain the reaction of ammonia with chlorine. Chlorine + Excess Ammonia Reaction of excess chlorine with less ammonia. A water system is having trouble maintaining a residual at the end of the distribution system. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the.. Chlorine + Excess Ammonia.
From cookinglove.com
Chlorine and ammonia Chlorine + Excess Ammonia The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. The residual leaving the plant is 2.5. The. Chlorine + Excess Ammonia.
From brainly.in
with excess chlorine, ammonia forms? Brainly.in Chlorine + Excess Ammonia Reaction of excess chlorine with less ammonia. The residual leaving the plant is 2.5. The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. Naocl + 2hcl → cl 2 +. When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. Ammonia. Chlorine + Excess Ammonia.
From www.toppr.com
Chlorine reacts with excess of ammonia to form Chlorine + Excess Ammonia Bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on the number of reactants taken for the reaction. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. The reaction of chlorine with excess of. Chlorine + Excess Ammonia.
From cookinglove.com
Chlorine and ammonia Chlorine + Excess Ammonia A water system is having trouble maintaining a residual at the end of the distribution system. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. Naocl + 2hcl → cl 2 +. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. Chlorine (cl 2) gas reacts. Chlorine + Excess Ammonia.
From www.doubtnut.com
Chlorine reacts with excess of ammonia to form. Chlorine + Excess Ammonia Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. Naocl + 2hcl → cl 2 +. When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. A water. Chlorine + Excess Ammonia.
From www.youtube.com
The reaction of liquid Ammonia and liquid Chlorine YouTube Chlorine + Excess Ammonia The reaction between ammonia and chlorine is much faster than the rate that chlorine kills bacteria so you cannot use chlorine to disinfect water that contains ammonia. Naocl + 2hcl → cl 2 +. A water system is having trouble maintaining a residual at the end of the distribution system. Ammonia reacts with chlorine to make chloramine (nh 2 cl),. Chlorine + Excess Ammonia.
From www.doubtnut.com
Ammonia reacts with excess of chlorine to form Chlorine + Excess Ammonia When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. The residual leaving the plant is 2.5. Bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. Nitrogen trichloride (. Chlorine + Excess Ammonia.
From www.doubtnut.com
Treatment of ammonia with excess of ethyl chloride will yield Chlorine + Excess Ammonia Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. Naocl + 2hcl → cl 2 +. The residual leaving the plant is 2.5. When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. A water system is having trouble maintaining a residual. Chlorine + Excess Ammonia.
From cookinglove.com
Chlorine and ammonia Chlorine + Excess Ammonia The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. Reaction of excess chlorine with less ammonia. When an operator has trouble maintaining constant combined residuals in plant processes or in the clearwell or in the. A water system is having trouble maintaining a residual at the end of the distribution. Chlorine + Excess Ammonia.
From askfilo.com
Ammonia reacts with excess of chlorine to form Filo Chlorine + Excess Ammonia The residual leaving the plant is 2.5. Bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on the number of reactants taken for the reaction. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. When an operator has. Chlorine + Excess Ammonia.
From www.toppr.com
Silver Chloride dissolves in excess ammonia due to the formation of a Chlorine + Excess Ammonia Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. Bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. Naocl + 2hcl → cl 2 +. Chlorine (cl 2) gas reacts with ammonia (nh 3). Chlorine + Excess Ammonia.
From cookinglove.com
Chlorine and ammonia Chlorine + Excess Ammonia The reaction between ammonia and chlorine is much faster than the rate that chlorine kills bacteria so you cannot use chlorine to disinfect water that contains ammonia. The reaction of chlorine with excess of ammonia results in the formation of ammonium chloride along with nitrogen gas. A water system is having trouble maintaining a residual at the end of the. Chlorine + Excess Ammonia.
From askfilo.com
(13.) Silver Chloride dissolves in excess ammonia due to the formation of.. Chlorine + Excess Ammonia Nitrogen trichloride ( ncl3 n c l 3 ) and hydrogen chloride vapour are formed when ammonia reacts. Ammonia reacts with chlorine to make chloramine (nh 2 cl), which is a gas. A water system is having trouble maintaining a residual at the end of the distribution system. Bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water.. Chlorine + Excess Ammonia.