Electron Configuration For Chlorine Ion . The chlorine atom has 17. See examples of common cations, anions, and polyatomic ions, and how they are named. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Add an electron to the anion electron configuration. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. See examples of sodium and chlorine ions, and how to. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. See the answer, the periodic table,.
from www.doubtnut.com
Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. The chlorine atom has 17. Add an electron to the anion electron configuration. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. See examples of sodium and chlorine ions, and how to. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. See the answer, the periodic table,. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. See examples of common cations, anions, and polyatomic ions, and how they are named.
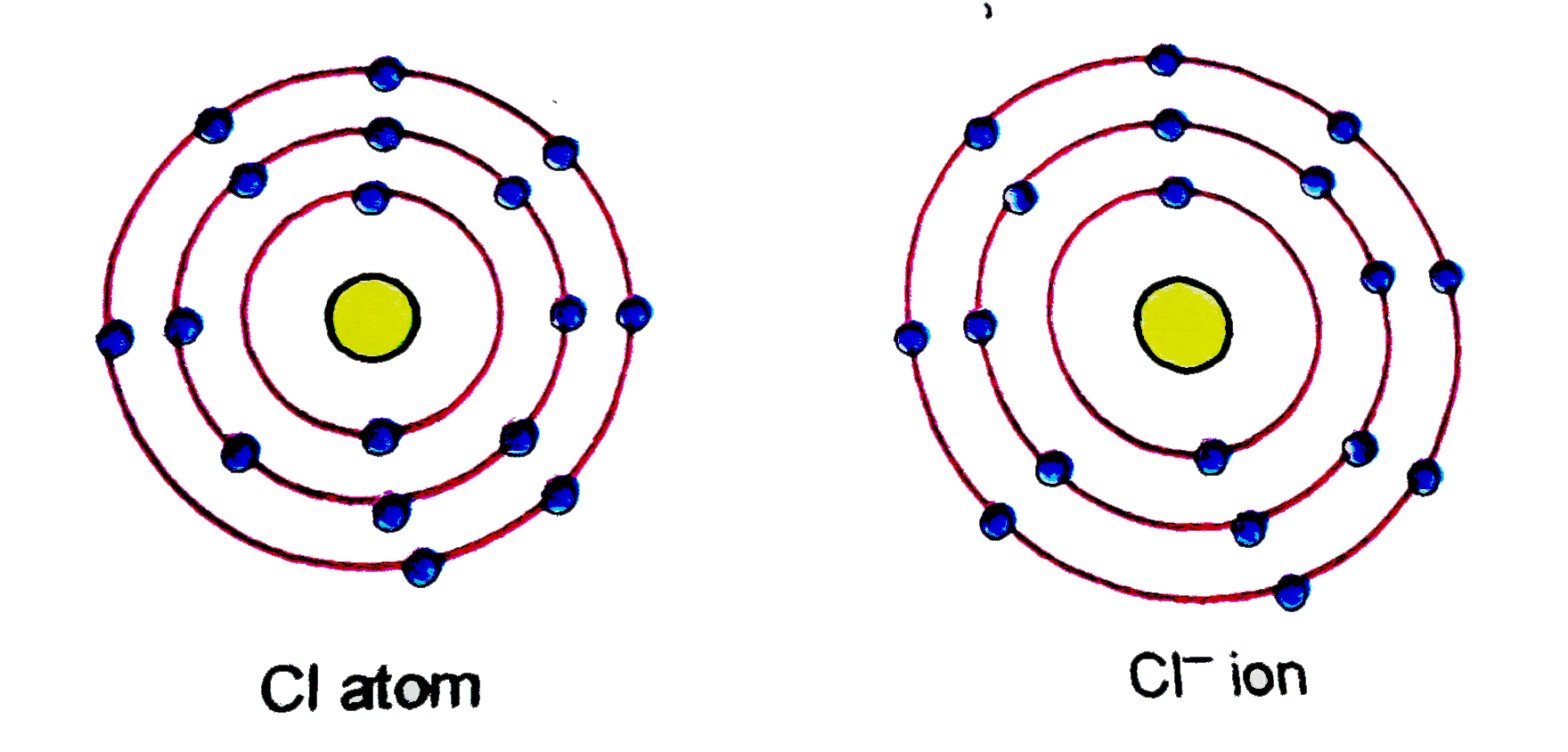
(a) Give the schematic atomic structures of chlorine atom and chloride
Electron Configuration For Chlorine Ion See examples of common cations, anions, and polyatomic ions, and how they are named. Add an electron to the anion electron configuration. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. See the answer, the periodic table,. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. See examples of sodium and chlorine ions, and how to. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. The chlorine atom has 17. See examples of common cations, anions, and polyatomic ions, and how they are named.
From dxopaibhs.blob.core.windows.net
A Chlorine Atom Gains An Electron. What Is The Resulting Particle at Electron Configuration For Chlorine Ion Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. See the answer, the periodic table,. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. The chlorine atom has 17. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. See. Electron Configuration For Chlorine Ion.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Electron Configuration For Chlorine Ion Add an electron to the anion electron configuration. The chlorine atom has 17. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. See examples of common cations, anions, and polyatomic ions, and how they are named. Learn how atoms form cations and anions by losing or gaining electrons to achieve an. Electron Configuration For Chlorine Ion.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Electron Configuration For Chlorine Ion Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. See examples of common cations, anions, and polyatomic ions, and how they are named. Add an electron to the anion electron configuration. The. Electron Configuration For Chlorine Ion.
From www.youtube.com
How to Draw the Lewis Dot Structure for Cl (Chloride ion) YouTube Electron Configuration For Chlorine Ion Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. The chlorine atom has 17. Add an electron to the anion electron configuration. See examples of sodium and chlorine ions, and how to. See the. Electron Configuration For Chlorine Ion.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Electron Configuration For Chlorine Ion For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. The chlorine atom has 17. Learn how to determine the electron configuration of cations and. Electron Configuration For Chlorine Ion.
From www.youtube.com
Chlorine Electron Configuration YouTube Electron Configuration For Chlorine Ion Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. Add an electron to the anion electron configuration. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. Learn how atoms form cations and anions by losing or gaining electrons. Electron Configuration For Chlorine Ion.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Electron Configuration For Chlorine Ion See examples of common cations, anions, and polyatomic ions, and how they are named. The chlorine atom has 17. See examples of sodium and chlorine ions, and how to. See the answer, the periodic table,. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. Learn how atoms form ions and ionic compounds by. Electron Configuration For Chlorine Ion.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Electron Configuration For Chlorine Ion Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. The. Electron Configuration For Chlorine Ion.
From 88guru.com
Electron Configuration Rules, Example and Diagram 88Guru Electron Configuration For Chlorine Ion Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. Add an electron to the anion electron configuration. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. See examples of common cations, anions, and polyatomic ions, and how they are named. The chlorine atom. Electron Configuration For Chlorine Ion.
From www.youtube.com
chloride ion electron configuration YouTube Electron Configuration For Chlorine Ion The chlorine atom has 17. Add an electron to the anion electron configuration. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. See examples of sodium and chlorine ions, and how to. See the answer, the periodic table,. Learn how to write the electron configuration for chlorine (cl). Electron Configuration For Chlorine Ion.
From www.animalia-life.club
Electron Configuration For Chlorine Electron Configuration For Chlorine Ion Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. The chlorine atom has 17. See examples of sodium and chlorine ions, and how to. See examples of common cations, anions, and polyatomic ions, and how they are named. Add an electron to the anion electron configuration. For example,. Electron Configuration For Chlorine Ion.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Electron Configuration For Chlorine Ion Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. The chlorine atom has 17. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to. Electron Configuration For Chlorine Ion.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Electron Configuration For Chlorine Ion See the answer, the periodic table,. See examples of sodium and chlorine ions, and how to. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. For example, the ground. Electron Configuration For Chlorine Ion.
From www.youtube.com
Writing Condensed/Abbreviated Electron Configuration for Chlorine (Cl Electron Configuration For Chlorine Ion See the answer, the periodic table,. The chlorine atom has 17. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify. Electron Configuration For Chlorine Ion.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Electron Configuration For Chlorine Ion Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. The chlorine atom has 17. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. Add an electron to the. Electron Configuration For Chlorine Ion.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Electron Configuration For Chlorine Ion For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. See the answer, the periodic table,. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. The chlorine atom has 17. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their. Electron Configuration For Chlorine Ion.
From 2012books.lardbucket.org
Ions Electron Configuration For Chlorine Ion Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. The chlorine atom has 17. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. Add an electron to the anion electron configuration. See examples of common cations, anions, and polyatomic ions, and. Electron Configuration For Chlorine Ion.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Electron Configuration For Chlorine Ion Add an electron to the anion electron configuration. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. See examples of sodium and chlorine ions, and how to. See the answer, the periodic table,. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their. Electron Configuration For Chlorine Ion.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Electron Configuration For Chlorine Ion See examples of common cations, anions, and polyatomic ions, and how they are named. See the answer, the periodic table,. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. See examples of sodium and chlorine ions, and how to.. Electron Configuration For Chlorine Ion.
From animalia-life.club
Lewis Dot Structure For Chlorine Electron Configuration For Chlorine Ion See examples of sodium and chlorine ions, and how to. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration. Electron Configuration For Chlorine Ion.
From pixels.com
Chlorine Electron Configuration Photograph by Electron Configuration For Chlorine Ion The chlorine atom has 17. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. See the answer, the periodic table,. Add an electron to the anion electron configuration. See examples of sodium and chlorine ions, and how to. Learn how to write the electron configuration for chlorine (cl) using the period. Electron Configuration For Chlorine Ion.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Electron Configuration For Chlorine Ion The chlorine atom has 17. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. See examples of sodium and chlorine ions, and how to. For example, the ground state electronic. Electron Configuration For Chlorine Ion.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Electron Configuration For Chlorine Ion See examples of common cations, anions, and polyatomic ions, and how they are named. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. The chlorine atom has 17. See the answer, the periodic table,. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. Learn. Electron Configuration For Chlorine Ion.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Electron Configuration For Chlorine Ion Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. Add an electron to the anion electron configuration. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. See examples of sodium and chlorine ions,. Electron Configuration For Chlorine Ion.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Electron Configuration For Chlorine Ion The chlorine atom has 17. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. See examples of sodium and chlorine ions, and how to. Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. See examples of common cations, anions, and. Electron Configuration For Chlorine Ion.
From joiyaimcz.blob.core.windows.net
Chlorine Valence Shell Electron Configuration at Emma Colburn blog Electron Configuration For Chlorine Ion Add an electron to the anion electron configuration. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. See the. Electron Configuration For Chlorine Ion.
From enginelistchester.z5.web.core.windows.net
Chlorine Electron Dot Diagram Electron Configuration For Chlorine Ion See examples of sodium and chlorine ions, and how to. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. See examples of common cations, anions, and polyatomic ions, and how they are named. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. Learn how atoms form cations and. Electron Configuration For Chlorine Ion.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Electron Configuration For Chlorine Ion See examples of common cations, anions, and polyatomic ions, and how they are named. See the answer, the periodic table,. The chlorine atom has 17. Add an electron to the anion electron configuration. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. See examples of sodium and chlorine ions, and how. Electron Configuration For Chlorine Ion.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Electron Configuration For Chlorine Ion See examples of common cations, anions, and polyatomic ions, and how they are named. See examples of sodium and chlorine ions, and how to. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. Learn how atoms form cations and anions by losing or gaining electrons to achieve an. Electron Configuration For Chlorine Ion.
From www.youtube.com
electronic configuration for Cl− (chloride ion)... k2chemistryclass Electron Configuration For Chlorine Ion For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. See examples of sodium and chlorine ions, and how to. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. The chlorine. Electron Configuration For Chlorine Ion.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Electron Configuration For Chlorine Ion The chlorine atom has 17. Add an electron to the anion electron configuration. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. See examples of common cations, anions, and polyatomic ions, and how they are named. Learn how atoms form cations and anions by losing or gaining electrons to achieve an. Electron Configuration For Chlorine Ion.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Electron Configuration For Chlorine Ion The chlorine atom has 17. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. See the answer, the periodic table,. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. See examples of common. Electron Configuration For Chlorine Ion.
From ar.inspiredpencil.com
Electron Configuration For Chlorine Electron Configuration For Chlorine Ion Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. See examples of sodium and chlorine ions, and how to. The chlorine atom has 17. See examples of common cations, anions, and polyatomic ions, and how they are named. Learn how to write the electron configuration for chlorine (cl) using the period table or. Electron Configuration For Chlorine Ion.
From sciencenotes.org
Chlorine Facts Electron Configuration For Chlorine Ion For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Learn how to write the electron configuration for chlorine (cl) using the period table or an electron configuration chart. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. Add an electron to the anion electron configuration. The. Electron Configuration For Chlorine Ion.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Electron Configuration For Chlorine Ion Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. See examples of sodium and chlorine ions, and how to. See examples of common cations, anions, and polyatomic ions, and how they are named. Learn how atoms form ions and ionic compounds by gaining or losing electrons to reach the octet rule. The chlorine. Electron Configuration For Chlorine Ion.