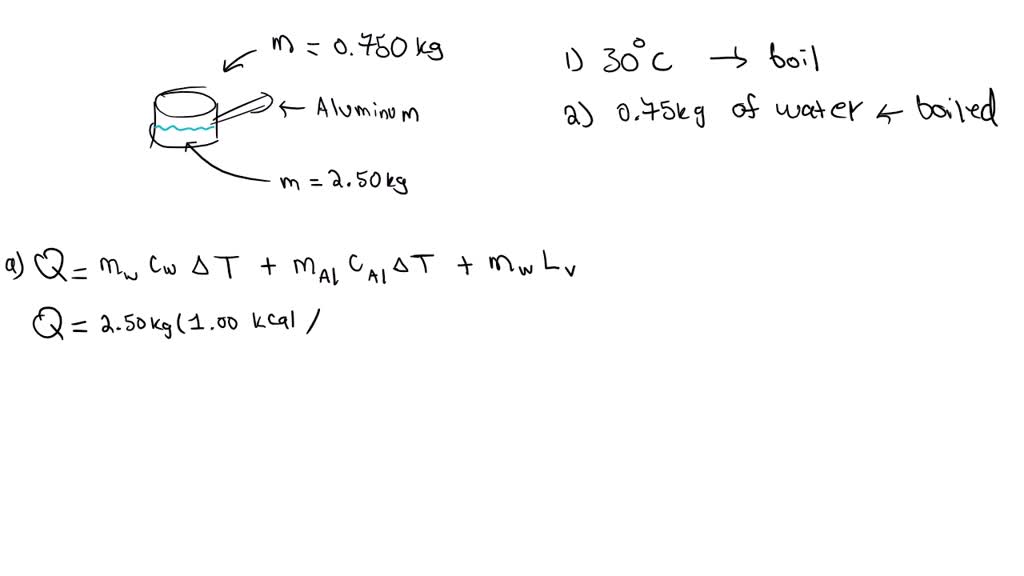Boiling Point Of Water Joules . The heat of vaporization of water is greater. It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. Water freezes at 0°c and boils at 100°c. Water is denser as a liquid than it is as a solid. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time. The specific heat of liquid water is greater than that of steam or ice. Here, too, the temperature remains constant at 100°c until all the water has been converted to steam. Since the density of water is 1 g/ml, 2 l of water is equal to 2000 g. To heat 1 g of water by 1 °c requires 4.184 j. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. When the temperature of the water reaches 100°c, the water begins to boil. Next, we calculate the temperature change by subtracting the initial. With our boiling point calculator, you can quickly determine the atmospheric boiling point of various substances.
from www.numerade.com
Since the density of water is 1 g/ml, 2 l of water is equal to 2000 g. The specific heat of liquid water is greater than that of steam or ice. It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. Water is denser as a liquid than it is as a solid. Here, too, the temperature remains constant at 100°c until all the water has been converted to steam. Water freezes at 0°c and boils at 100°c. When the temperature of the water reaches 100°c, the water begins to boil. To heat 1 g of water by 1 °c requires 4.184 j. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. The heat of vaporization of water is greater.
(a) How much heat transfer is required to raise the temperature of a 0.
Boiling Point Of Water Joules To heat 1 g of water by 1 °c requires 4.184 j. Water is denser as a liquid than it is as a solid. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time. Water freezes at 0°c and boils at 100°c. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. The heat of vaporization of water is greater. With our boiling point calculator, you can quickly determine the atmospheric boiling point of various substances. The specific heat of liquid water is greater than that of steam or ice. Since the density of water is 1 g/ml, 2 l of water is equal to 2000 g. Next, we calculate the temperature change by subtracting the initial. It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. When the temperature of the water reaches 100°c, the water begins to boil. To heat 1 g of water by 1 °c requires 4.184 j. Here, too, the temperature remains constant at 100°c until all the water has been converted to steam.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Boiling Point Of Water Joules Water freezes at 0°c and boils at 100°c. Since the density of water is 1 g/ml, 2 l of water is equal to 2000 g. With our boiling point calculator, you can quickly determine the atmospheric boiling point of various substances. The heat of vaporization of water is greater. Our water heating calculator can help you determine both the amount. Boiling Point Of Water Joules.
From byjus.com
64. The boiling point of water at 735 torr is 99.07 degree Celsius the Boiling Point Of Water Joules Here, too, the temperature remains constant at 100°c until all the water has been converted to steam. The specific heat of liquid water is greater than that of steam or ice. Next, we calculate the temperature change by subtracting the initial. Water freezes at 0°c and boils at 100°c. When the temperature of the water reaches 100°c, the water begins. Boiling Point Of Water Joules.
From www.numerade.com
SOLVEDUsing Figure 10.6 and the specific heat of water, 4.184 J / g ^∘ Boiling Point Of Water Joules The specific heat of liquid water is greater than that of steam or ice. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. Next, we calculate the temperature change by subtracting the initial. It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling. Boiling Point Of Water Joules.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Boiling Point Of Water Joules To heat 1 g of water by 1 °c requires 4.184 j. Water freezes at 0°c and boils at 100°c. Water is denser as a liquid than it is as a solid. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time. When the. Boiling Point Of Water Joules.
From www.numerade.com
SOLVED kettle containing 7kg of water has just reached it boiling Boiling Point Of Water Joules Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. Water freezes at 0°c and boils at 100°c. Water is denser as a liquid than it is as a solid. The heat of vaporization of water is greater. Our water heating calculator can help you determine both the amount of heat. Boiling Point Of Water Joules.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Boiling Point Of Water Joules Water is denser as a liquid than it is as a solid. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. The specific heat. Boiling Point Of Water Joules.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Boiling Point Of Water Joules Water freezes at 0°c and boils at 100°c. Next, we calculate the temperature change by subtracting the initial. The specific heat of liquid water is greater than that of steam or ice. Since the density of water is 1 g/ml, 2 l of water is equal to 2000 g. To heat 1 g of water by 1 °c requires 4.184. Boiling Point Of Water Joules.
From www.quora.com
Does the boiling point of water increase as we move towards outer space Boiling Point Of Water Joules The heat of vaporization of water is greater. With our boiling point calculator, you can quickly determine the atmospheric boiling point of various substances. Here, too, the temperature remains constant at 100°c until all the water has been converted to steam. Water is denser as a liquid than it is as a solid. To heat 1 g of water by. Boiling Point Of Water Joules.
From www.animalia-life.club
Boiling Point Of Water Examples Boiling Point Of Water Joules Water freezes at 0°c and boils at 100°c. Water is denser as a liquid than it is as a solid. The heat of vaporization of water is greater. Next, we calculate the temperature change by subtracting the initial. The specific heat of liquid water is greater than that of steam or ice. Since we have 2 l of water, which. Boiling Point Of Water Joules.
From www.numerade.com
SOLVED Constants for freezingpoint depression and boilingpoint Boiling Point Of Water Joules Since the density of water is 1 g/ml, 2 l of water is equal to 2000 g. Here, too, the temperature remains constant at 100°c until all the water has been converted to steam. Water is denser as a liquid than it is as a solid. When the temperature of the water reaches 100°c, the water begins to boil. It. Boiling Point Of Water Joules.
From byjus.com
64. The boiling point of water at 735 torr is 99.07 degree Celsius the Boiling Point Of Water Joules Water freezes at 0°c and boils at 100°c. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time. Since the density of water is 1 g/ml, 2 l of water is equal to 2000 g. Water is denser as a liquid than it is. Boiling Point Of Water Joules.
From nl.dreamstime.com
Kookpunt Van Water Op Verschillende Hoogtemeterniveaus Vector Boiling Point Of Water Joules It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. With our boiling point calculator, you can quickly determine the atmospheric boiling point of various substances. Here, too, the temperature remains constant at 100°c until all the water has been converted to steam. To heat 1. Boiling Point Of Water Joules.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 Boiling Point Of Water Joules To heat 1 g of water by 1 °c requires 4.184 j. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. Here, too, the temperature remains. Boiling Point Of Water Joules.
From lyndajguilleno.blob.core.windows.net
What Happens To Water When It Reaches Its Boiling Point at Boiling Point Of Water Joules To heat 1 g of water by 1 °c requires 4.184 j. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. With our boiling point calculator, you can quickly determine the atmospheric boiling point of various substances. The heat of vaporization of water is greater. Water is denser as a. Boiling Point Of Water Joules.
From www.numerade.com
(a) How much heat transfer is required to raise the temperature of a 0. Boiling Point Of Water Joules Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time. Here, too, the temperature remains constant at 100°c until all the water has been converted. Boiling Point Of Water Joules.
From exodhbolj.blob.core.windows.net
What Is The Boiling Point Of Liquid In Celsius at Felecia Greenwald blog Boiling Point Of Water Joules It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. Next, we calculate the temperature change by subtracting the initial. Since the density of water is 1 g/ml, 2 l of water is equal to 2000 g. Our water heating calculator can help you determine both. Boiling Point Of Water Joules.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Boiling Point Of Water Joules The specific heat of liquid water is greater than that of steam or ice. When the temperature of the water reaches 100°c, the water begins to boil. Water is denser as a liquid than it is as a solid. To heat 1 g of water by 1 °c requires 4.184 j. The heat of vaporization of water is greater. Next,. Boiling Point Of Water Joules.
From marshallkruwmathis.blogspot.com
What is the Boiling Point of Water MarshallkruwMathis Boiling Point Of Water Joules Water freezes at 0°c and boils at 100°c. When the temperature of the water reaches 100°c, the water begins to boil. The heat of vaporization of water is greater. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time. The specific heat of liquid. Boiling Point Of Water Joules.
From exyqxtcdu.blob.core.windows.net
What Is The Boiling Point Constant Of Water at Beatrice Hughes blog Boiling Point Of Water Joules The specific heat of liquid water is greater than that of steam or ice. Next, we calculate the temperature change by subtracting the initial. Water is denser as a liquid than it is as a solid. When the temperature of the water reaches 100°c, the water begins to boil. Water freezes at 0°c and boils at 100°c. With our boiling. Boiling Point Of Water Joules.
From byjus.com
boiling point of water at 750 mm Hg is 99.63 ^oC .How much sugar is to Boiling Point Of Water Joules To heat 1 g of water by 1 °c requires 4.184 j. The specific heat of liquid water is greater than that of steam or ice. Water freezes at 0°c and boils at 100°c. The heat of vaporization of water is greater. Since the density of water is 1 g/ml, 2 l of water is equal to 2000 g. When. Boiling Point Of Water Joules.
From blog.thermoworks.com
High Altitude and Its Effects on Cooking Boiling Point Of Water Joules It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. Here, too, the temperature remains constant at 100°c until all the water has been converted to steam. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its.. Boiling Point Of Water Joules.
From brainly.com
This graph shows the melting and boiling points of the alkali metals Boiling Point Of Water Joules The heat of vaporization of water is greater. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time. The specific heat of liquid water is greater than that of steam or ice. Since we have 2 l of water, which is equivalent to 2000. Boiling Point Of Water Joules.
From www.worksheetsplanet.com
What is Boiling Point Boiling Point Of Water Joules Next, we calculate the temperature change by subtracting the initial. It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. Water freezes at 0°c and boils at. Boiling Point Of Water Joules.
From www.animalia-life.club
Boiling Point Of Water For Kids Boiling Point Of Water Joules When the temperature of the water reaches 100°c, the water begins to boil. With our boiling point calculator, you can quickly determine the atmospheric boiling point of various substances. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. Next, we calculate the temperature change by subtracting the initial. The specific. Boiling Point Of Water Joules.
From facts.net
20 Fascinating Facts About Boiling Point Boiling Point Of Water Joules Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. When the temperature of the water reaches 100°c, the water begins to boil. Since the density of water is 1 g/ml, 2 l of water is equal to 2000 g. Our water heating calculator can help you determine both the amount. Boiling Point Of Water Joules.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin Boiling Point Of Water Joules Here, too, the temperature remains constant at 100°c until all the water has been converted to steam. It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. To heat 1 g of water by 1 °c requires 4.184 j. Water is denser as a liquid than. Boiling Point Of Water Joules.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Boiling Point Of Water Joules With our boiling point calculator, you can quickly determine the atmospheric boiling point of various substances. Here, too, the temperature remains constant at 100°c until all the water has been converted to steam. Water is denser as a liquid than it is as a solid. Since we have 2 l of water, which is equivalent to 2000 g, and we. Boiling Point Of Water Joules.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Boiling Point Of Water Joules It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time. Since the density of water is 1 g/ml, 2 l. Boiling Point Of Water Joules.
From bceweb.org
Water Boiling Chart A Visual Reference of Charts Chart Master Boiling Point Of Water Joules With our boiling point calculator, you can quickly determine the atmospheric boiling point of various substances. The heat of vaporization of water is greater. Water freezes at 0°c and boils at 100°c. Next, we calculate the temperature change by subtracting the initial. When the temperature of the water reaches 100°c, the water begins to boil. To heat 1 g of. Boiling Point Of Water Joules.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes Boiling Point Of Water Joules It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time. The specific heat of liquid water is greater than that. Boiling Point Of Water Joules.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Boiling Point Of Water Joules Next, we calculate the temperature change by subtracting the initial. Here, too, the temperature remains constant at 100°c until all the water has been converted to steam. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time. When the temperature of the water reaches. Boiling Point Of Water Joules.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry Boiling Point Of Water Joules Water freezes at 0°c and boils at 100°c. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. To heat 1 g of water by 1 °c requires 4.184 j. Next, we calculate the temperature change by subtracting the initial. Since the density of water is 1 g/ml, 2 l of. Boiling Point Of Water Joules.
From www.numerade.com
SOLVED Calculate the total heat in joules needed to convert 32.0 g of Boiling Point Of Water Joules It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. The specific heat of liquid water is greater than that of steam or ice. When the temperature of the water reaches 100°c, the water begins to boil. Our water heating calculator can help you determine both. Boiling Point Of Water Joules.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free Boiling Point Of Water Joules Since the density of water is 1 g/ml, 2 l of water is equal to 2000 g. It takes approximately 4186 joules to raise the temperature of one liter of water to its boiling point and an additional 2260 joules to. To heat 1 g of water by 1 °c requires 4.184 j. When the temperature of the water reaches. Boiling Point Of Water Joules.
From joiblqdxc.blob.core.windows.net
Does River Water Have Minerals at Esteban Hines blog Boiling Point Of Water Joules To heat 1 g of water by 1 °c requires 4.184 j. Here, too, the temperature remains constant at 100°c until all the water has been converted to steam. Since we have 2 l of water, which is equivalent to 2000 g, and we want to raise its. With our boiling point calculator, you can quickly determine the atmospheric boiling. Boiling Point Of Water Joules.