Standard Reduction Potential And Spontaneity . The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The overall cell potential is the reduction potential of the reductive half. They are measured in volts, and they tell you how. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Standard reduction potentials are very useful in chemistry. Describe and relate the definitions of electrode and cell potentials. By the end of this section, you will be able to: Interpret electrode potentials in terms of relative oxidant and reductant. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. They are also known as standard cell potentials, or standard electrode potentials. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire.
from www.numerade.com
The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Interpret electrode potentials in terms of relative oxidant and reductant. They are also known as standard cell potentials, or standard electrode potentials. They are measured in volts, and they tell you how. Standard reduction potentials are very useful in chemistry. The overall cell potential is the reduction potential of the reductive half. Describe and relate the definitions of electrode and cell potentials. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials.
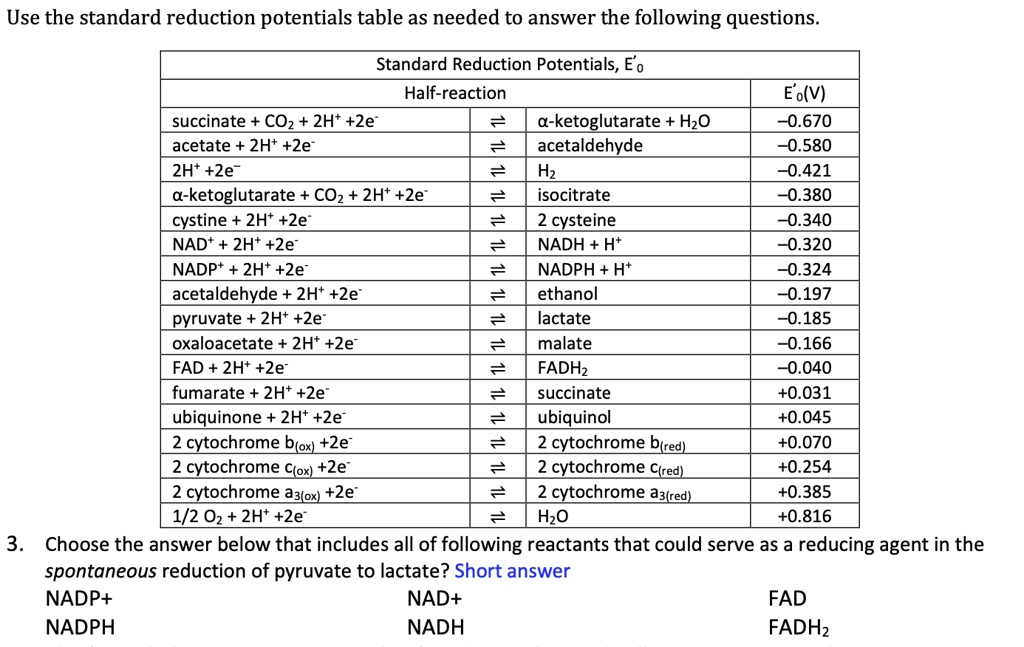
SOLVED Use the standard reduction potentials table as needed to answer
Standard Reduction Potential And Spontaneity By the end of this section, you will be able to: The overall cell potential is the reduction potential of the reductive half. They are measured in volts, and they tell you how. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Describe and relate the definitions of electrode and cell potentials. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. By the end of this section, you will be able to: The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. Interpret electrode potentials in terms of relative oxidant and reductant. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Standard reduction potentials are very useful in chemistry. They are also known as standard cell potentials, or standard electrode potentials.
From www.slideserve.com
PPT 19.2 Galvanic Cells 19.3 Standard Reduction Potentials 19.4 Standard Reduction Potential And Spontaneity The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Describe and relate the definitions of electrode and cell potentials. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices.. Standard Reduction Potential And Spontaneity.
From www.numerade.com
SOLVEDA chemist designs a galvanic cell that uses these two half Standard Reduction Potential And Spontaneity They are measured in volts, and they tell you how. The overall cell potential is the reduction potential of the reductive half. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. Describe. Standard Reduction Potential And Spontaneity.
From www.chegg.com
Solved I. Reduction Potential And Reaction Spontaneity A.... Standard Reduction Potential And Spontaneity The overall cell potential is the reduction potential of the reductive half. Describe and relate the definitions of electrode and cell potentials. They are measured in volts, and they tell you how. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. By convention, all tabulated values. Standard Reduction Potential And Spontaneity.
From www.numerade.com
SOLVED Question 2 (1 point) Given the following half reactions with Standard Reduction Potential And Spontaneity Standard reduction potentials are very useful in chemistry. Interpret electrode potentials in terms of relative oxidant and reductant. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. They are also known as standard cell potentials, or standard electrode potentials. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that. Standard Reduction Potential And Spontaneity.
From www.bartleby.com
Answered Based on the information in the table… bartleby Standard Reduction Potential And Spontaneity Standard reduction potentials are very useful in chemistry. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The overall cell potential is the reduction potential of the reductive half. Describe and relate the definitions. Standard Reduction Potential And Spontaneity.
From www.numerade.com
SOLVED 1 Calculate the standard cell potential (from the Standard Standard Reduction Potential And Spontaneity Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. Describe and relate the definitions of electrode and cell potentials. They are also known as standard cell potentials, or standard electrode potentials. By the end of this section, you will be able to: Interpret electrode potentials in terms of relative oxidant. Standard Reduction Potential And Spontaneity.
From vtxidsdgzc.blogspot.com
How To Calculate Reduction Potential Of Half Reaction Besides simply Standard Reduction Potential And Spontaneity By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Interpret electrode potentials in terms of relative oxidant and reductant. They are also known as standard cell potentials, or standard electrode potentials. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. They are measured in. Standard Reduction Potential And Spontaneity.
From dxoasuand.blob.core.windows.net
Calculate The Standard Cell Potentials Given The Following Data at Standard Reduction Potential And Spontaneity By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. By the end of this section, you will be able to: The overall cell potential is the reduction potential of the reductive half. Standard reduction potentials are. Standard Reduction Potential And Spontaneity.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential And Spontaneity By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. By the end of this section, you will be able to: The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Describe and relate the definitions of electrode and cell potentials. Figure. Standard Reduction Potential And Spontaneity.
From www.numerade.com
SOLVED The standard potential for the renction Ln ZAg" Zn 2Ag Is [.56 Standard Reduction Potential And Spontaneity They are also known as standard cell potentials, or standard electrode potentials. They are measured in volts, and they tell you how. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Standard reduction potentials are very useful in chemistry. By convention, all tabulated values of standard electrode potentials are listed as. Standard Reduction Potential And Spontaneity.
From www.chegg.com
Solved StANDARD REDUCTION PotentialS AND Associated Standard Standard Reduction Potential And Spontaneity Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Describe and relate the definitions of electrode and cell potentials. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. They are. Standard Reduction Potential And Spontaneity.
From www.flinnsci.ca
Standard Reduction Potential Chart Flinn Scientific Standard Reduction Potential And Spontaneity The overall cell potential is the reduction potential of the reductive half. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential, also known as the standard electrode potential,. Standard Reduction Potential And Spontaneity.
From www.numerade.com
SOLVED Selective Reduction The standard reduction potential for the Standard Reduction Potential And Spontaneity Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. They are also known as standard cell potentials, or standard electrode potentials. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. They are measured in volts, and they tell you how. The standard. Standard Reduction Potential And Spontaneity.
From f15.beauty
Standard Reduction Potential Table Standard Reduction Potential And Spontaneity They are also known as standard cell potentials, or standard electrode potentials. By the end of this section, you will be able to: By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. They are measured in volts,. Standard Reduction Potential And Spontaneity.
From ch302.cm.utexas.edu
Standard Reduction Potentials Standard Reduction Potential And Spontaneity By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. They are measured in volts, and they tell you how. Describe and relate the definitions of electrode and cell potentials. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The overall cell potential is the reduction potential. Standard Reduction Potential And Spontaneity.
From www.numerade.com
SOLVED 08 spontaneity 4 Points Which pairing of species will Standard Reduction Potential And Spontaneity Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. The overall cell potential is the reduction potential of the reductive half. By the end of this section, you will be able to: The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at.. Standard Reduction Potential And Spontaneity.
From www.youtube.com
19.1.4 Predict whether a reaction will be spontaneous using standard Standard Reduction Potential And Spontaneity The overall cell potential is the reduction potential of the reductive half. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. They are also known as standard cell potentials, or standard electrode potentials. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species. Standard Reduction Potential And Spontaneity.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Magnesium Standard Reduction Potential And Spontaneity The overall cell potential is the reduction potential of the reductive half. By the end of this section, you will be able to: Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at.. Standard Reduction Potential And Spontaneity.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential And Spontaneity Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. By the end of this section, you will be able to: They are also known as standard cell. Standard Reduction Potential And Spontaneity.
From www.chegg.com
Solved 1) Consider the following standard reduction Standard Reduction Potential And Spontaneity The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The overall cell potential is the reduction potential of the reductive half. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. Use standard reduction potentials to determine the better oxidizing or reducing. Standard Reduction Potential And Spontaneity.
From www.coursehero.com
. Standard Reduction Potentials at 25 C (298 K) for Many Common Standard Reduction Potential And Spontaneity Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Standard reduction potentials are very useful in chemistry. They are also known as standard cell potentials, or. Standard Reduction Potential And Spontaneity.
From www.numerade.com
SOLVED Use the standard reduction potentials table as needed to answer Standard Reduction Potential And Spontaneity They are also known as standard cell potentials, or standard electrode potentials. By the end of this section, you will be able to: The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. By convention, all tabulated values of standard electrode potentials are listed as standard reduction. Standard Reduction Potential And Spontaneity.
From chemistrytalk.org
Standard Reduction Potentials made easy ChemTalk Standard Reduction Potential And Spontaneity Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. The overall cell potential is the reduction potential of. Standard Reduction Potential And Spontaneity.
From www.chem.fsu.edu
Lab Purpose Standard Reduction Potential And Spontaneity Standard reduction potentials are very useful in chemistry. Describe and relate the definitions of electrode and cell potentials. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential can be determined by subtracting the standard reduction potential for the. Standard Reduction Potential And Spontaneity.
From www.youtube.com
19.1 Cell potential and Gibbs free energy (HL) YouTube Standard Reduction Potential And Spontaneity The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Standard reduction potentials are very useful in chemistry. By the end of this section, you will be able to: Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on. Standard Reduction Potential And Spontaneity.
From www.numerade.com
SOLVED Selective Oxidation The standard reduction potential for the Standard Reduction Potential And Spontaneity Interpret electrode potentials in terms of relative oxidant and reductant. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on. Standard Reduction Potential And Spontaneity.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4831905 Standard Reduction Potential And Spontaneity By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. They are measured in volts, and they tell you how. By the end of this section, you will be able to: The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Standard reduction potentials are very useful. Standard Reduction Potential And Spontaneity.
From www.slideserve.com
PPT Electrochemistry Part IV Spontaneity & Nernst Equation Standard Reduction Potential And Spontaneity The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Standard reduction potentials are very useful in chemistry. Describe and relate the definitions of electrode and cell potentials. By the end of this section, you will be able to: By convention, all tabulated values of standard electrode potentials are listed as standard. Standard Reduction Potential And Spontaneity.
From www.chegg.com
Solved 8. Explain how to use the standard reduction Standard Reduction Potential And Spontaneity Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. The overall cell potential is the reduction potential of the reductive half. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. By convention, all tabulated values of standard electrode potentials are listed as. Standard Reduction Potential And Spontaneity.
From www.numerade.com
SOLVED Assignment 6.4 Calculating Standard Reduction Potentials (15 Standard Reduction Potential And Spontaneity The overall cell potential is the reduction potential of the reductive half. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Interpret electrode potentials in terms of. Standard Reduction Potential And Spontaneity.
From www.youtube.com
2. Standard Reduction Potentials and Reaction Spontaneity YouTube Standard Reduction Potential And Spontaneity The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based. Standard Reduction Potential And Spontaneity.
From www.chegg.com
Solved using the standard reduction potentials Determine if Standard Reduction Potential And Spontaneity Interpret electrode potentials in terms of relative oxidant and reductant. By the end of this section, you will be able to: The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is,. Standard Reduction Potential And Spontaneity.
From www.slideserve.com
PPT 19.2 Galvanic Cells 19.3 Standard Reduction Potentials 19.4 Standard Reduction Potential And Spontaneity By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Describe and relate the definitions of electrode and cell potentials.. Standard Reduction Potential And Spontaneity.
From loensgcfn.blob.core.windows.net
Standard Reduction Potential Calculator at James Burkley blog Standard Reduction Potential And Spontaneity Figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the system—that is, based on the. They are also known as standard cell potentials, or standard electrode potentials. The overall cell potential is the reduction potential of the reductive half. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices.. Standard Reduction Potential And Spontaneity.
From www.slideserve.com
PPT Electrochemistry Part III Reduction Potentials PowerPoint Standard Reduction Potential And Spontaneity They are measured in volts, and they tell you how. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Standard reduction potentials are very useful in chemistry. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. They are also known as standard cell potentials, or. Standard Reduction Potential And Spontaneity.