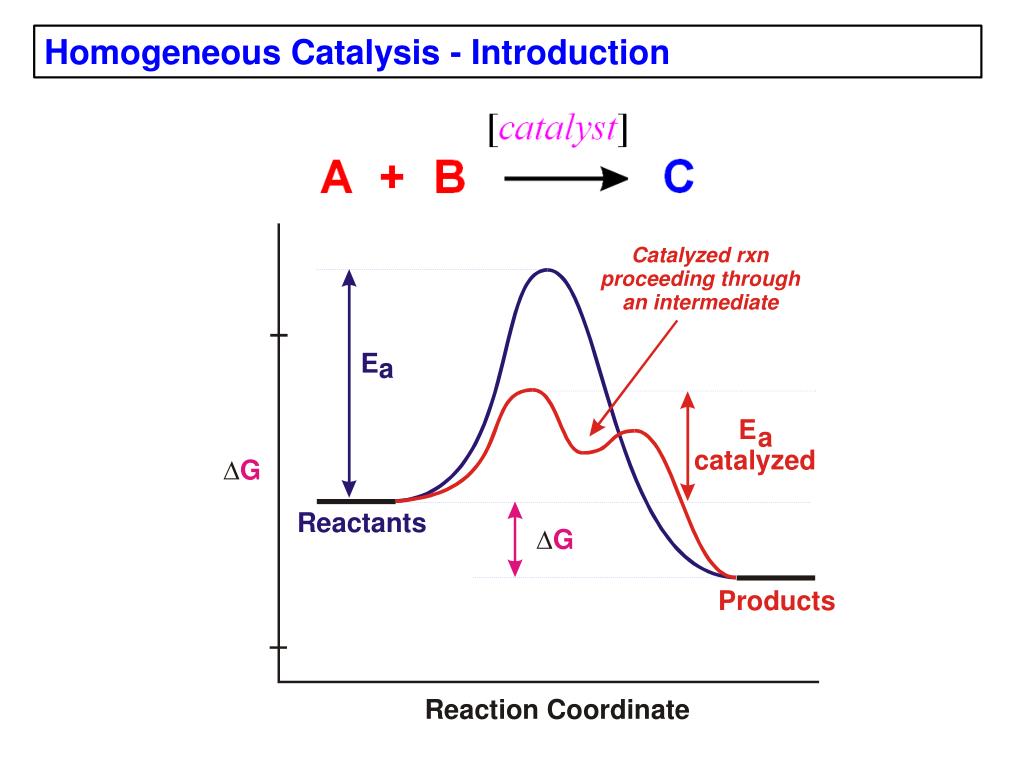
Catalysis Examples . In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. A very small amount of catalyst is required to alter the reaction rate. A catalyzed reaction is typically used to accelerate the rate by which specific chemistry is to proceed. How to burn a sugar cube; This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Platinum as an oxidation catalyst; The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Some common examples of catalysis. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In homogeneous catalysis, catalysts are in the same phase as the reactants. Catalysis is the process of speeding up a reaction using a catalyst. Enzymes are biological catalysts that. What is a catalyzed reaction?
from www.slideserve.com
In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Enzymes are biological catalysts that. In homogeneous catalysis, catalysts are in the same phase as the reactants. A catalyzed reaction is typically used to accelerate the rate by which specific chemistry is to proceed. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A very small amount of catalyst is required to alter the reaction rate. What is a catalyzed reaction? The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction.
PPT Homogeneous Catalysis Introduction PowerPoint Presentation
Catalysis Examples A very small amount of catalyst is required to alter the reaction rate. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Some common examples of catalysis. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. A very small amount of catalyst is required to alter the reaction rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Platinum as an oxidation catalyst; How to burn a sugar cube; In homogeneous catalysis, catalysts are in the same phase as the reactants. Catalysis is the process of speeding up a reaction using a catalyst. What is a catalyzed reaction? A catalyzed reaction is typically used to accelerate the rate by which specific chemistry is to proceed. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalysis Examples What is a catalyzed reaction? Catalysis is the process of speeding up a reaction using a catalyst. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are biological catalysts that. Some common examples of catalysis. Catalysis. Catalysis Examples.
From www.slideserve.com
PPT Nanocatalyst PowerPoint Presentation, free download ID676158 Catalysis Examples How to burn a sugar cube; Some common examples of catalysis. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Platinum as an oxidation catalyst; Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. A catalyzed reaction is typically used. Catalysis Examples.
From www.slideshare.net
Catalysis Catalysis Examples Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis is the process of speeding up a reaction using a catalyst. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. The word “catalyst” comes from the greek word kataluein, which means to loosen. Catalysis Examples.
From www.essentialchemicalindustry.org
Catalysis in industry Catalysis Examples Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of. Catalysis Examples.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysis Examples Enzymes are biological catalysts that. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A very small amount of catalyst is required to alter the reaction rate. Catalysts allow. Catalysis Examples.
From www.slideshare.net
Catalysis Catalysis Examples The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. What is a catalyzed reaction? A very small amount of catalyst is required to alter the reaction rate. In homogeneous catalysis, catalysts are in the same. Catalysis Examples.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation Catalysis Examples Catalysis is the process of speeding up a reaction using a catalyst. Platinum as an oxidation catalyst; Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. A catalyzed reaction is. Catalysis Examples.
From www.britannica.com
Catalysis Chemistry, Classification, & Chemical Reactions Britannica Catalysis Examples In homogeneous catalysis, catalysts are in the same phase as the reactants. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis is the process of speeding up a reaction using a catalyst. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance. Catalysis Examples.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalysis Examples The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Enzymes are biological catalysts that. A catalyzed reaction is typically used to accelerate the rate by which specific chemistry is to proceed.. Catalysis Examples.
From alchetron.com
Heterogeneous catalysis Alchetron, the free social encyclopedia Catalysis Examples Platinum as an oxidation catalyst; In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysis is the process of speeding up a reaction using a catalyst. Some common examples of catalysis. What is a catalyzed reaction? In homogeneous catalysis, catalysts are in the same phase as the. Catalysis Examples.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalysis Examples The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction using a catalyst. What is a catalyzed reaction? A very small amount of catalyst is required to alter the reaction rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. Catalysis Examples.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalysis Examples The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. In homogeneous catalysis, catalysts are in the same phase as the reactants. Catalysis is the process of speeding up a reaction using a catalyst. A very small amount of catalyst is required to alter the reaction rate. Catalysts allow a reaction to proceed via a. Catalysis Examples.
From 2012books.lardbucket.org
Catalysis Catalysis Examples Some common examples of catalysis. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. How to burn a sugar cube; The word “catalyst” comes from the greek word kataluein, which means to loosen or. Catalysis Examples.
From www.slideserve.com
PPT Homogeneous Catalysis Introduction PowerPoint Presentation Catalysis Examples What is a catalyzed reaction? Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In homogeneous catalysis, catalysts are in the same phase as the reactants. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. A. Catalysis Examples.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalysis Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysis is the process of speeding up a reaction using a catalyst. In homogeneous catalysis, catalysts are in the same phase as the reactants. Catalyst, in chemistry, any substance that increases the rate of a reaction. Catalysis Examples.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalysis Examples Some common examples of catalysis. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In homogeneous catalysis, catalysts are in the same phase as the reactants. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Platinum as an oxidation. Catalysis Examples.
From slidetodoc.com
Chapter 23 Catalysis in Organic Reactions and in Catalysis Examples Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A very small amount of catalyst is required to alter the reaction rate. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. The word “catalyst” comes from the greek word kataluein,. Catalysis Examples.
From www.youtube.com
01.09 General Acid Catalysis YouTube Catalysis Examples A very small amount of catalyst is required to alter the reaction rate. Catalysis is the process of speeding up a reaction using a catalyst. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. What is a catalyzed reaction? How to burn a sugar cube; Catalysis is a process of. Catalysis Examples.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysis Examples Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. How. Catalysis Examples.
From www.slideserve.com
PPT Chapter 15 Enzymatic Catalysis PowerPoint Presentation, free Catalysis Examples How to burn a sugar cube; A catalyzed reaction is typically used to accelerate the rate by which specific chemistry is to proceed. A very small amount of catalyst is required to alter the reaction rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. What is a catalyzed reaction? This page looks. Catalysis Examples.
From www.pinterest.com
Catalyst Easy Science Energy activities, Chemical reactions Catalysis Examples Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are biological catalysts that. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. A catalyzed reaction is typically used to accelerate the rate by which specific chemistry is to proceed. Catalysis is a process of increasing the. Catalysis Examples.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysis Examples In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Some common examples of catalysis. What is a catalyzed reaction? How to burn a sugar cube; A very small amount of catalyst is required to alter the reaction rate. Catalyst, in chemistry, any substance that increases the rate. Catalysis Examples.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalysis Examples What is a catalyzed reaction? Enzymes are biological catalysts that. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysis is the process of speeding up a reaction using a catalyst. Platinum as an oxidation catalyst; A catalyzed reaction is typically used to accelerate the rate by. Catalysis Examples.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysis Examples A very small amount of catalyst is required to alter the reaction rate. What is a catalyzed reaction? Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Enzymes are biological catalysts that. Catalysts allow a reaction to proceed via a pathway that has a lower activation. Catalysis Examples.
From www.cademix.org
Applications of Heterogeneous Catalysis in Industry Catalysis Examples Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Platinum as an oxidation catalyst; A catalyzed reaction is typically used to accelerate the rate by which specific chemistry is to proceed. In chemistry and biology, a catalyst is a substance the increases the rate of a. Catalysis Examples.
From www.slideshare.net
Heterogeneous catalysisFundamentals Catalysis Examples A very small amount of catalyst is required to alter the reaction rate. Platinum as an oxidation catalyst; This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Enzymes are biological catalysts that. What is a catalyzed reaction? Catalyst, in chemistry, any substance that increases the. Catalysis Examples.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysis Examples How to burn a sugar cube; Some common examples of catalysis. Enzymes are biological catalysts that. A very small amount of catalyst is required to alter the reaction rate. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. In homogeneous catalysis, catalysts are in the. Catalysis Examples.
From www.chemistrystudent.com
Homogeneous Catalysis (ALevel) ChemistryStudent Catalysis Examples How to burn a sugar cube; Enzymes are biological catalysts that. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyzed. Catalysis Examples.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalysis Examples Catalysis is the process of speeding up a reaction using a catalyst. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. In homogeneous catalysis, catalysts are in the same phase as the reactants. Catalyst, in chemistry, any substance that increases the rate of a reaction without. Catalysis Examples.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalysis Examples What is a catalyzed reaction? A catalyzed reaction is typically used to accelerate the rate by which specific chemistry is to proceed. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. Catalysis Examples.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalysis Examples A very small amount of catalyst is required to alter the reaction rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyzed reaction is typically used to accelerate the rate by which specific chemistry is to proceed. Platinum as an oxidation catalyst; In chemistry and biology, a catalyst is a substance. Catalysis Examples.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalysis Examples What is a catalyzed reaction? Platinum as an oxidation catalyst; How to burn a sugar cube; In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. In homogeneous catalysis, catalysts are in. Catalysis Examples.
From www.slideserve.com
PPT Enzyme Catalysis PowerPoint Presentation, free download ID2683708 Catalysis Examples Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process of speeding up a reaction. Catalysis Examples.
From www.slideserve.com
PPT Enzyme Catalysis PowerPoint Presentation, free download ID6835497 Catalysis Examples How to burn a sugar cube; The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Enzymes are biological catalysts that. What is a catalyzed reaction? This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Some common examples of catalysis.. Catalysis Examples.
From www.slideshare.net
Catalyst Catalysis Examples In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. How to burn a sugar cube; Enzymes are biological catalysts that. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. This page looks at the the different types of catalyst. Catalysis Examples.