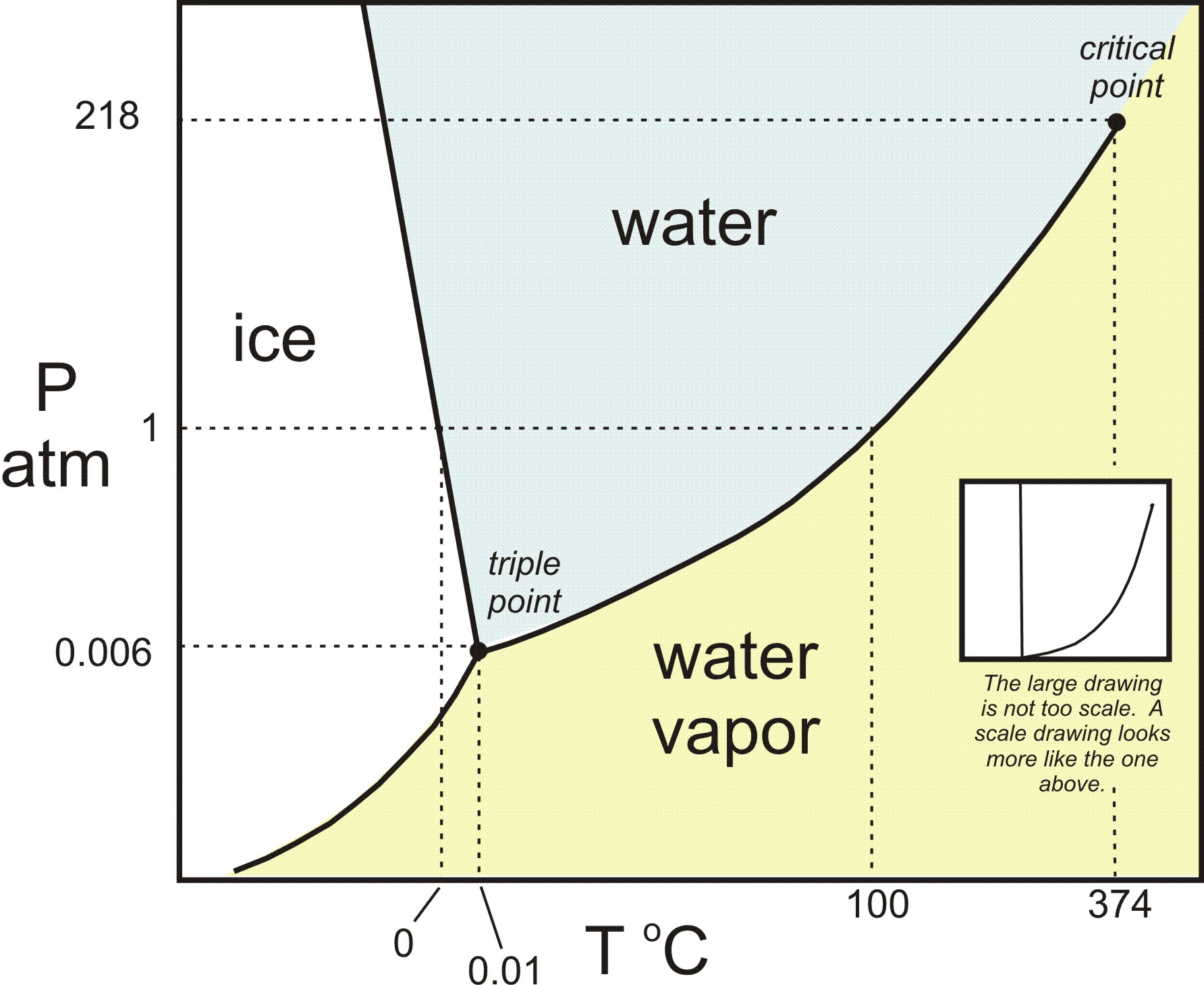
Does Water Evaporate Faster At Higher Altitudes . This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Since this is a naturally. Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in the southern hemisphere, and ecmwf was 16% faster in. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. This means that at 100°c, you can have pure water vapor at atmospheric pressure. More energy, as in higher heat, makes molecules move even faster. Pure or distilled water evaporates faster than saltwater and other types of impure water. The rate of horizontal water movement was also skewed: Water molecules have an easy time escaping off the surface when the air pressure above them is less.
from scienceline.ucsb.edu
Water molecules have an easy time escaping off the surface when the air pressure above them is less. Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in the southern hemisphere, and ecmwf was 16% faster in. Since this is a naturally. This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. Pure or distilled water evaporates faster than saltwater and other types of impure water. This means that at 100°c, you can have pure water vapor at atmospheric pressure. More energy, as in higher heat, makes molecules move even faster.
UCSB Science Line
Does Water Evaporate Faster At Higher Altitudes Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in the southern hemisphere, and ecmwf was 16% faster in. The rate of horizontal water movement was also skewed: Pure or distilled water evaporates faster than saltwater and other types of impure water. Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. Water molecules have an easy time escaping off the surface when the air pressure above them is less. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). This means that at 100°c, you can have pure water vapor at atmospheric pressure. This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. Since this is a naturally. Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in the southern hemisphere, and ecmwf was 16% faster in. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. More energy, as in higher heat, makes molecules move even faster.
From slideplayer.com
AIM DO NOW HW. ppt download Does Water Evaporate Faster At Higher Altitudes This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. The rate of horizontal water movement was also skewed: One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Merra had water moving 106% faster in the northern hemisphere and. Does Water Evaporate Faster At Higher Altitudes.
From www.slideserve.com
PPT Splish! Splash! WATER! PowerPoint Presentation, free download Does Water Evaporate Faster At Higher Altitudes Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in the southern hemisphere, and ecmwf was 16% faster in. The rate of horizontal water movement was also skewed: This means that at 100°c, you can have pure water vapor at atmospheric pressure. One definition of boiling point is the temperature at which the substance's. Does Water Evaporate Faster At Higher Altitudes.
From www.worldatlas.com
The Water Cycle WorldAtlas Does Water Evaporate Faster At Higher Altitudes Since this is a naturally. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. This means that at 100°c, you can have pure water vapor at atmospheric pressure. More energy, as in higher. Does Water Evaporate Faster At Higher Altitudes.
From slideplayer.com
CHAPTER 9 Liquids and Solids ppt video online download Does Water Evaporate Faster At Higher Altitudes Pure or distilled water evaporates faster than saltwater and other types of impure water. More energy, as in higher heat, makes molecules move even faster. The rate of horizontal water movement was also skewed: Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in the southern hemisphere, and ecmwf was 16% faster in. When. Does Water Evaporate Faster At Higher Altitudes.
From pressbooks.umn.edu
Water Cycle Classroom Partners Does Water Evaporate Faster At Higher Altitudes This means that at 100°c, you can have pure water vapor at atmospheric pressure. This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more. Does Water Evaporate Faster At Higher Altitudes.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does Water Evaporate Faster At Higher Altitudes Since this is a naturally. More energy, as in higher heat, makes molecules move even faster. Pure or distilled water evaporates faster than saltwater and other types of impure water. This means that at 100°c, you can have pure water vapor at atmospheric pressure. Water molecules have an easy time escaping off the surface when the air pressure above them. Does Water Evaporate Faster At Higher Altitudes.
From www.slideshare.net
Evaporation Does Water Evaporate Faster At Higher Altitudes This means that at 100°c, you can have pure water vapor at atmospheric pressure. Since this is a naturally. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Water molecules have an easy time escaping off the surface when the air pressure above them is less. Pure or distilled water. Does Water Evaporate Faster At Higher Altitudes.
From www.waterev.com
The Water Cycle Definition! Easy Science Lesson for Kids Does Water Evaporate Faster At Higher Altitudes More energy, as in higher heat, makes molecules move even faster. This means that at 100°c, you can have pure water vapor at atmospheric pressure. This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. One definition of boiling point is the temperature at which the substance's vapour pressure. Does Water Evaporate Faster At Higher Altitudes.
From slideplayer.com
Atmospheric Moisture. ppt download Does Water Evaporate Faster At Higher Altitudes When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Water molecules have an easy time escaping off the surface when the air pressure above them is less. Since this is a naturally. More energy, as in higher heat, makes molecules move even faster. This means that at 100°c, you can have pure. Does Water Evaporate Faster At Higher Altitudes.
From slideplayer.com
The Nature of Liquids. ppt download Does Water Evaporate Faster At Higher Altitudes Pure or distilled water evaporates faster than saltwater and other types of impure water. Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in the southern hemisphere, and ecmwf was 16% faster in. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Since this is a. Does Water Evaporate Faster At Higher Altitudes.
From fyokqptpd.blob.core.windows.net
How Quickly Does Water Evaporate at Delores Kessler blog Does Water Evaporate Faster At Higher Altitudes Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. The rate of horizontal water movement was also skewed:. Does Water Evaporate Faster At Higher Altitudes.
From www.pinterest.com
Adjusting For Altitude Pressure Canning in 2022 Pressure canning Does Water Evaporate Faster At Higher Altitudes This means that at 100°c, you can have pure water vapor at atmospheric pressure. Water molecules have an easy time escaping off the surface when the air pressure above them is less. This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. Pure or distilled water evaporates faster than. Does Water Evaporate Faster At Higher Altitudes.
From www.slideserve.com
PPT The Water Cycle PowerPoint Presentation, free download ID9418730 Does Water Evaporate Faster At Higher Altitudes Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. Pure or distilled water evaporates faster than saltwater and other types of impure water. Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in. Does Water Evaporate Faster At Higher Altitudes.
From www.slideserve.com
PPT Water in the Atmosphere PowerPoint Presentation, free download Does Water Evaporate Faster At Higher Altitudes Water molecules have an easy time escaping off the surface when the air pressure above them is less. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. The rate of horizontal water movement. Does Water Evaporate Faster At Higher Altitudes.
From scienceblogs.com
Water in Space What Happens? ScienceBlogs Does Water Evaporate Faster At Higher Altitudes This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. Pure or distilled water evaporates faster than saltwater and other types of impure water. Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more. Does Water Evaporate Faster At Higher Altitudes.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country Does Water Evaporate Faster At Higher Altitudes Water molecules have an easy time escaping off the surface when the air pressure above them is less. More energy, as in higher heat, makes molecules move even faster. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Saltwater has another substance dissolved in it (salt), so its particles attach. Does Water Evaporate Faster At Higher Altitudes.
From www.pinterest.com
Model of air pressure and how it acts. 05PS11 Molecule diagram Does Water Evaporate Faster At Higher Altitudes This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. Water molecules have an easy time escaping off the surface when the air pressure above them is less. More energy, as in higher heat, makes molecules move even faster. Saltwater has another substance dissolved in it (salt), so its. Does Water Evaporate Faster At Higher Altitudes.
From slideplayer.com
Properties of Water Polarity Cohesion Adhesion Solubility ppt download Does Water Evaporate Faster At Higher Altitudes More energy, as in higher heat, makes molecules move even faster. Since this is a naturally. Water molecules have an easy time escaping off the surface when the air pressure above them is less. The rate of horizontal water movement was also skewed: Pure or distilled water evaporates faster than saltwater and other types of impure water. Merra had water. Does Water Evaporate Faster At Higher Altitudes.
From slideplayer.com
Chapter 11 ( ) Intermolecular Forces, Liquids and Solids ppt download Does Water Evaporate Faster At Higher Altitudes Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Pure or distilled water evaporates faster than saltwater and other types of impure. Does Water Evaporate Faster At Higher Altitudes.
From www.slideserve.com
PPT Water States of Matter PowerPoint Presentation, free download Does Water Evaporate Faster At Higher Altitudes One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Pure or distilled water evaporates faster than saltwater and other types of impure water. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. This means that at 100°c, you can have pure water. Does Water Evaporate Faster At Higher Altitudes.
From young6science3.weebly.com
5Th grade science The Water Cycle Does Water Evaporate Faster At Higher Altitudes Water molecules have an easy time escaping off the surface when the air pressure above them is less. Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. Since this is a naturally. This means that at 100°c, you can. Does Water Evaporate Faster At Higher Altitudes.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Does Water Evaporate Faster At Higher Altitudes When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Water molecules have an easy time escaping off the surface when the air pressure above them is less. Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in the southern hemisphere, and ecmwf was 16% faster in.. Does Water Evaporate Faster At Higher Altitudes.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point Does Water Evaporate Faster At Higher Altitudes This means that at 100°c, you can have pure water vapor at atmospheric pressure. Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. More energy, as in higher heat, makes molecules move even faster. This is why water boils. Does Water Evaporate Faster At Higher Altitudes.
From exouqimxj.blob.core.windows.net
Does The Water Boil Faster With Lid On It at Domingo Rouse blog Does Water Evaporate Faster At Higher Altitudes Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in the southern hemisphere, and ecmwf was 16% faster in. This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. Saltwater has another substance dissolved in it (salt), so its particles attach themselves to. Does Water Evaporate Faster At Higher Altitudes.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does Water Evaporate Faster At Higher Altitudes This means that at 100°c, you can have pure water vapor at atmospheric pressure. Pure or distilled water evaporates faster than saltwater and other types of impure water. The rate of horizontal water movement was also skewed: Since this is a naturally. Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making. Does Water Evaporate Faster At Higher Altitudes.
From fyokqptpd.blob.core.windows.net
How Quickly Does Water Evaporate at Delores Kessler blog Does Water Evaporate Faster At Higher Altitudes The rate of horizontal water movement was also skewed: Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water.. Does Water Evaporate Faster At Higher Altitudes.
From scienceline.ucsb.edu
UCSB Science Line Does Water Evaporate Faster At Higher Altitudes Water molecules have an easy time escaping off the surface when the air pressure above them is less. The rate of horizontal water movement was also skewed: Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. Merra had water. Does Water Evaporate Faster At Higher Altitudes.
From socratic.org
How does atmospheric pressure change with altitude? Socratic Does Water Evaporate Faster At Higher Altitudes Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in the southern hemisphere, and ecmwf was 16% faster in. Water molecules have an easy time escaping off the surface when the air pressure above them is less. More energy, as in higher heat, makes molecules move even faster. This means that at 100°c, you. Does Water Evaporate Faster At Higher Altitudes.
From slideplayer.com
Chemistry I Unit 9 The Gas Laws Text Questions from Wilbraham, et. al Does Water Evaporate Faster At Higher Altitudes Water molecules have an easy time escaping off the surface when the air pressure above them is less. This means that at 100°c, you can have pure water vapor at atmospheric pressure. The rate of horizontal water movement was also skewed: Pure or distilled water evaporates faster than saltwater and other types of impure water. This is why water boils. Does Water Evaporate Faster At Higher Altitudes.
From www.youtube.com
Evaporation Water Evaporates Faster When Temperature Is High science Does Water Evaporate Faster At Higher Altitudes This means that at 100°c, you can have pure water vapor at atmospheric pressure. More energy, as in higher heat, makes molecules move even faster. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Merra had water moving 106% faster in the northern hemisphere and up to 45% slower in the southern. Does Water Evaporate Faster At Higher Altitudes.
From www.wave3.com
Behind the Forecast Outwitting the weather when baking Does Water Evaporate Faster At Higher Altitudes One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Since this is a naturally. Water molecules have an easy time escaping off the surface when the air pressure above them is less. This is why water boils at 100°c at sea level—a bubble of steam can form below the surface. Does Water Evaporate Faster At Higher Altitudes.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Does Water Evaporate Faster At Higher Altitudes When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. Pure or distilled water evaporates faster than saltwater and other types of impure. Does Water Evaporate Faster At Higher Altitudes.
From masterconceptsinchemistry.com
How does the vapor pressure of solution depend on the concentration of Does Water Evaporate Faster At Higher Altitudes Water molecules have an easy time escaping off the surface when the air pressure above them is less. Since this is a naturally. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). This is why water boils at 100°c at sea level—a bubble of steam can form below the surface. Does Water Evaporate Faster At Higher Altitudes.
From slideplayer.com
The Particle Model and Changes of State ppt download Does Water Evaporate Faster At Higher Altitudes When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. This is why water boils at 100°c at sea level—a bubble of steam can form below the surface of the water. Since this is a naturally. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside. Does Water Evaporate Faster At Higher Altitudes.
From www.teachoo.com
Why is Evaporation called a surface phenomenon? Teachoo Does Water Evaporate Faster At Higher Altitudes Saltwater has another substance dissolved in it (salt), so its particles attach themselves to the water molecules, making them heavier and in need of more energy to escape the surface. Pure or distilled water evaporates faster than saltwater and other types of impure water. More energy, as in higher heat, makes molecules move even faster. Since this is a naturally.. Does Water Evaporate Faster At Higher Altitudes.