Copper Hydroxide Temperature . It decomposes at temperatures above. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. Reactions of hexaaquacopper (ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium hydroxide solution) remove. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. But isn't it a solid by itself too?
from www.semanticscholar.org
Hydroxide ions (from, say, sodium hydroxide solution) remove. Reactions of hexaaquacopper (ii) ions with hydroxide ions. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. It decomposes at temperatures above. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. But isn't it a solid by itself too?
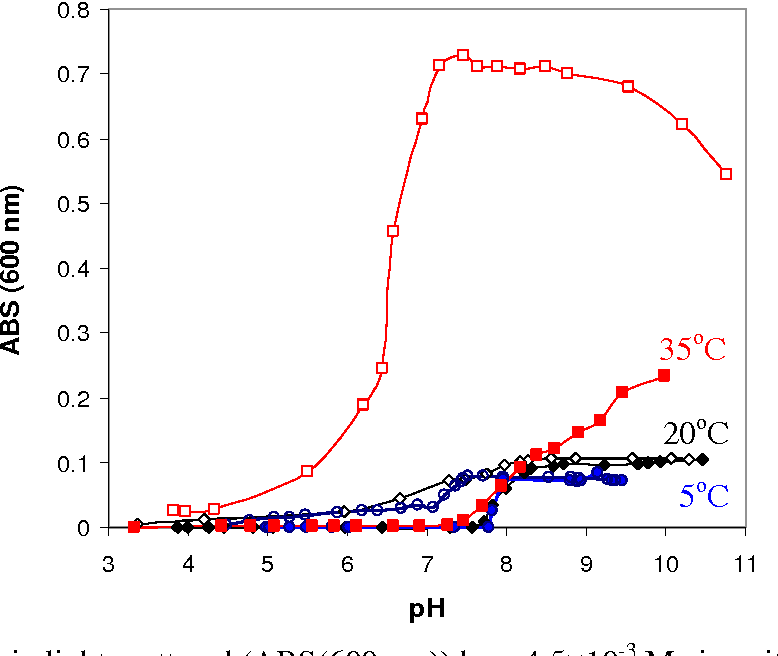
Effect of pH, concentration and temperature on copper and zinc
Copper Hydroxide Temperature It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Hydroxide ions (from, say, sodium hydroxide solution) remove. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. But isn't it a solid by itself too? So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. It decomposes at temperatures above. Reactions of hexaaquacopper (ii) ions with hydroxide ions. It is slightly soluble in water and more soluble in acids or ammonium hydroxide.
From www.fishersci.ca
Cupric Hydroxide, Technical, Spectrum™ Chemical Fisher Scientific Copper Hydroxide Temperature The solubility of copper(ii) hydroxide generally increases with an increase in temperature. But isn't it a solid by itself too? Hydroxide ions (from, say, sodium hydroxide solution) remove. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. It is slightly soluble in water and more soluble in acids or ammonium. Copper Hydroxide Temperature.
From steploced.weebly.com
How to read copper pourbaix diagram steploced Copper Hydroxide Temperature Hydroxide ions (from, say, sodium hydroxide solution) remove. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. It decomposes at temperatures above. But isn't it a solid by itself too? It is slightly soluble in water and more. Copper Hydroxide Temperature.
From www.semanticscholar.org
Figure 1 from Effect of pH, concentration and temperature on copper and Copper Hydroxide Temperature So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Reactions of hexaaquacopper (ii) ions with hydroxide ions. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Hydroxide ions (from, say, sodium hydroxide solution) remove. When it comes to temperature, copper(ii) hydroxide hangs. Copper Hydroxide Temperature.
From www.youtube.com
Making copper hydroxide YouTube Copper Hydroxide Temperature When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. But isn't it a solid by itself too? It decomposes at temperatures above. Hydroxide ions (from, say, sodium hydroxide solution) remove. Copper(ii) hydroxide reacts with a solution of ammonia. Copper Hydroxide Temperature.
From www.sciencephoto.com
Copper hydroxide precipitate Stock Image A500/0411 Science Photo Copper Hydroxide Temperature When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. It decomposes at temperatures above. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. But isn't it a solid by itself too? It is slightly soluble in. Copper Hydroxide Temperature.
From www.chegg.com
Solved The copper (11) hydroxide system equilibrium is Copper Hydroxide Temperature When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. Hydroxide ions (from, say, sodium hydroxide solution) remove. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+. Copper Hydroxide Temperature.
From www.semanticscholar.org
Effect of pH, concentration and temperature on copper and zinc Copper Hydroxide Temperature But isn't it a solid by itself too? Reactions of hexaaquacopper (ii) ions with hydroxide ions. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Hydroxide ions (from, say, sodium hydroxide solution) remove. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. It is slightly soluble in water and more soluble in acids or ammonium. Copper Hydroxide Temperature.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(OH)2 = CuO + H2O YouTube Copper Hydroxide Temperature Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Reactions of hexaaquacopper (ii) ions with hydroxide ions. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits. Copper Hydroxide Temperature.
From www.researchgate.net
6. Solubility versus pH curves for the thermodynamically stable Copper Hydroxide Temperature But isn't it a solid by itself too? Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. It decomposes at temperatures above. When it comes to temperature, copper(ii) hydroxide hangs out in the. Copper Hydroxide Temperature.
From www.youtube.com
4_Copper(II) hydroxide + Heat YouTube Copper Hydroxide Temperature Hydroxide ions (from, say, sodium hydroxide solution) remove. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. It decomposes at temperatures above. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the. Copper Hydroxide Temperature.
From www.slideserve.com
PPT Evidence of Chemical Change Laboratory PowerPoint Presentation Copper Hydroxide Temperature When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Reactions of hexaaquacopper (ii) ions with hydroxide ions. But isn't it a solid by itself too? Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution. Copper Hydroxide Temperature.
From www.semanticscholar.org
Figure 9 from Effect of pH, concentration and temperature on copper and Copper Hydroxide Temperature Reactions of hexaaquacopper (ii) ions with hydroxide ions. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. When. Copper Hydroxide Temperature.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Temperature It decomposes at temperatures above. Hydroxide ions (from, say, sodium hydroxide solution) remove. But isn't it a solid by itself too? The solubility of copper(ii) hydroxide generally increases with an increase in temperature. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. It is slightly soluble in water and more. Copper Hydroxide Temperature.
From www.scribd.com
Reactions of Copper Redox Sodium Hydroxide Copper Hydroxide Temperature It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Reactions of hexaaquacopper (ii) ions with hydroxide ions. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Hydroxide ions (from, say, sodium hydroxide solution) remove. But isn't it a solid by itself too? When it comes to temperature, copper(ii) hydroxide hangs out in the. Copper Hydroxide Temperature.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper Hydroxide Temperature It is slightly soluble in water and more soluble in acids or ammonium hydroxide. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. Hydroxide ions (from, say, sodium hydroxide solution) remove. So just wondering, copper(ii) hydroxide undergoes thermal. Copper Hydroxide Temperature.
From www.youtube.com
Precipitation Reactions Copper Hydroxide Senior YouTube Copper Hydroxide Temperature Reactions of hexaaquacopper (ii) ions with hydroxide ions. It decomposes at temperatures above. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. Hydroxide ions (from, say, sodium hydroxide solution) remove. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. The solubility of copper(ii) hydroxide generally. Copper Hydroxide Temperature.
From www.slideserve.com
PPT Evidence of Chemical Change Laboratory PowerPoint Presentation Copper Hydroxide Temperature Reactions of hexaaquacopper (ii) ions with hydroxide ions. But isn't it a solid by itself too? Hydroxide ions (from, say, sodium hydroxide solution) remove. It decomposes at temperatures above. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. When it comes to temperature, copper(ii) hydroxide hangs. Copper Hydroxide Temperature.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper Hydroxide Temperature Hydroxide ions (from, say, sodium hydroxide solution) remove. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. But isn't it a solid by itself too?. Copper Hydroxide Temperature.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper Hydroxide Temperature But isn't it a solid by itself too? Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits. Copper Hydroxide Temperature.
From www.semanticscholar.org
Figure 2 from Effect of pH, concentration and temperature on copper and Copper Hydroxide Temperature When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. Hydroxide ions (from, say, sodium hydroxide solution) remove. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. It decomposes at temperatures above. But isn't. Copper Hydroxide Temperature.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Copper Hydroxide Temperature It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. It decomposes at temperatures above. Hydroxide ions (from, say, sodium hydroxide solution) remove. Reactions of hexaaquacopper (ii) ions with hydroxide ions.. Copper Hydroxide Temperature.
From www.researchgate.net
FESEM images of copper tin hydroxide nanoparticles prepared by Copper Hydroxide Temperature So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Hydroxide ions (from, say, sodium hydroxide solution) remove. But isn't it a solid by itself too? Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. It is slightly soluble in water and more. Copper Hydroxide Temperature.
From www.youtube.com
Make copper hydroxide with just two compounds YouTube Copper Hydroxide Temperature It decomposes at temperatures above. Reactions of hexaaquacopper (ii) ions with hydroxide ions. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. It is slightly. Copper Hydroxide Temperature.
From www.semanticscholar.org
Figure 4 from Effect of pH, concentration and temperature on copper and Copper Hydroxide Temperature Reactions of hexaaquacopper (ii) ions with hydroxide ions. But isn't it a solid by itself too? It is slightly soluble in water and more soluble in acids or ammonium hydroxide. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90. Copper Hydroxide Temperature.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Temperature Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Hydroxide ions (from, say, sodium hydroxide solution) remove. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state. Copper Hydroxide Temperature.
From www.researchgate.net
Post annealing temperaturedependent morphological and electrochemical Copper Hydroxide Temperature It is slightly soluble in water and more soluble in acids or ammonium hydroxide. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. But isn't it a solid by itself too? So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Hydroxide ions (from, say, sodium hydroxide solution) remove.. Copper Hydroxide Temperature.
From www.ceramic-glazes.com
Copper Hydroxide Cupric hydroxide patina for ceramics Copper Hydroxide Temperature It decomposes at temperatures above. Hydroxide ions (from, say, sodium hydroxide solution) remove. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. But isn't it a solid by itself too? So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. When it. Copper Hydroxide Temperature.
From www.researchgate.net
Solubility curves for common metals in freshwater with pH [60 Copper Hydroxide Temperature Hydroxide ions (from, say, sodium hydroxide solution) remove. It decomposes at temperatures above. The solubility of copper(ii) hydroxide generally increases with an increase in temperature. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. But isn't it a solid by itself too? Reactions of hexaaquacopper (ii) ions with hydroxide ions. Copper(ii) hydroxide reacts with a solution of ammonia. Copper Hydroxide Temperature.
From bnrc.springeropen.com
Copper(II) oxide nanocatalyst preparation and characterization green Copper Hydroxide Temperature It is slightly soluble in water and more soluble in acids or ammonium hydroxide. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. But isn't it a solid by itself too? When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. It decomposes at temperatures above. Copper(ii) hydroxide reacts. Copper Hydroxide Temperature.
From www.semanticscholar.org
Effect of pH, concentration and temperature on copper and zinc Copper Hydroxide Temperature Reactions of hexaaquacopper (ii) ions with hydroxide ions. But isn't it a solid by itself too? When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. Hydroxide ions (from, say, sodium hydroxide solution) remove. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper(ii) hydroxide reacts. Copper Hydroxide Temperature.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube Copper Hydroxide Temperature But isn't it a solid by itself too? It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. It decomposes at temperatures above. When it comes to temperature, copper(ii) hydroxide hangs. Copper Hydroxide Temperature.
From www.pdfprof.com
PDF copper hydroxide PDF Télécharger Download Copper Hydroxide Temperature Hydroxide ions (from, say, sodium hydroxide solution) remove. It decomposes at temperatures above. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. It is slightly. Copper Hydroxide Temperature.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Copper Hydroxide Temperature When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. But isn't it a solid by itself too? So just wondering, copper(ii) hydroxide undergoes thermal decomposition. Copper Hydroxide Temperature.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Temperature Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3) 4] 2+ complex ion, but the. Reactions of hexaaquacopper (ii) ions with hydroxide ions. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. So just wondering, copper(ii) hydroxide undergoes thermal decomposition. Copper Hydroxide Temperature.
From www.sciencephoto.com
Copper Hydroxide Precipitate Stock Image C027/9441 Science Photo Copper Hydroxide Temperature But isn't it a solid by itself too? It is slightly soluble in water and more soluble in acids or ammonium hydroxide. When it comes to temperature, copper(ii) hydroxide hangs out in the solid state until it hits around 90 °c. Copper(ii) hydroxide reacts with a solution of ammonia to form a deep blue solution consisting of the [cu(nh 3). Copper Hydroxide Temperature.