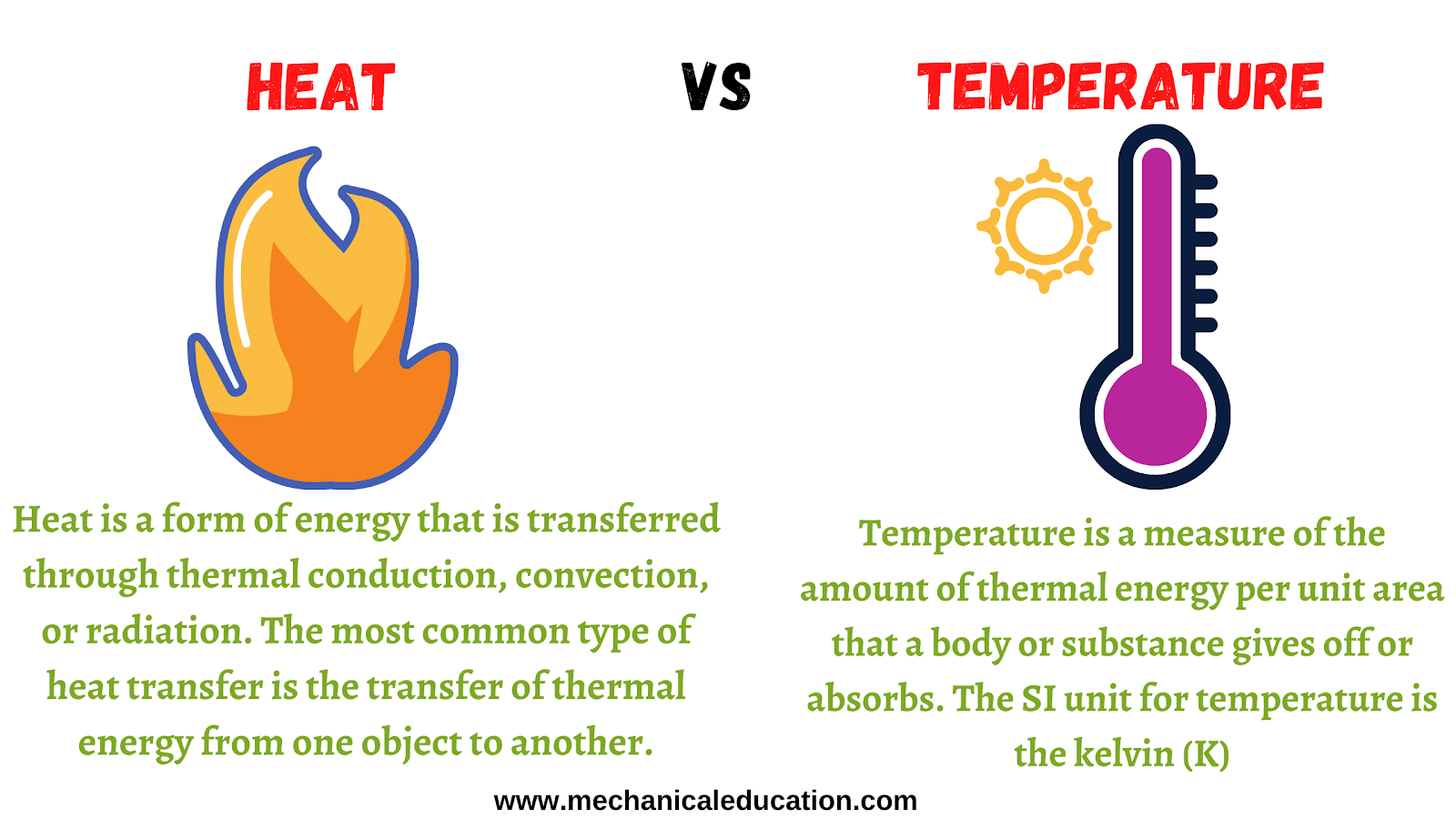
Heat Definition Thermal . Thermal energy is the energy due to the motion of atoms and molecules in a substance. All matter contains heat energy, and the more heat energy that is present, the hotter an item or area will be. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Heat, energy that is transferred from one body to another as the result of a difference in temperature. In thermodynamics, heat means energy which is. Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. This flow of heat continues until the two objects reach the same temperature. It accounts for translational, vibrational, and rotational motion. We have defined work as force times distance and learned that work done on an object changes its kinetic. If two bodies at different temperatures are brought together, energy is. Define heat as transfer of energy. More simply put, heat energy, also called thermal energy or simply heat, is transferred from one location to another by particles bouncing into each other. As long as the temperature difference is maintained, a flow of heat will occur.
from www.mechanicaleducation.com
We have defined work as force times distance and learned that work done on an object changes its kinetic. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Thermal energy is the energy due to the motion of atoms and molecules in a substance. More simply put, heat energy, also called thermal energy or simply heat, is transferred from one location to another by particles bouncing into each other. In thermodynamics, heat means energy which is. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. It accounts for translational, vibrational, and rotational motion. All matter contains heat energy, and the more heat energy that is present, the hotter an item or area will be.
Difference between Heat and Temperature Mechanical Education
Heat Definition Thermal Heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. All matter contains heat energy, and the more heat energy that is present, the hotter an item or area will be. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. We have defined work as force times distance and learned that work done on an object changes its kinetic. If two bodies at different temperatures are brought together, energy is. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. In thermodynamics, heat means energy which is. This flow of heat continues until the two objects reach the same temperature. More simply put, heat energy, also called thermal energy or simply heat, is transferred from one location to another by particles bouncing into each other. It accounts for translational, vibrational, and rotational motion. Define heat as transfer of energy. Thermal energy is the energy due to the motion of atoms and molecules in a substance. As long as the temperature difference is maintained, a flow of heat will occur.
From exoxuekni.blob.core.windows.net
What Are The Types Of Thermal Energy at James Winston blog Heat Definition Thermal If two bodies at different temperatures are brought together, energy is. Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. This flow of heat continues until the two objects reach the same temperature. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. All matter contains heat energy, and the more heat. Heat Definition Thermal.
From cameronbutler6.blogspot.com
Heat and Temperature Cameron Butler Heat Definition Thermal Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. This flow of heat continues until the two objects reach the same temperature. All matter contains heat energy, and the more heat energy that is present, the hotter an item or area will. Heat Definition Thermal.
From sciencenotes.org
What Is Temperature? Definition in Science Heat Definition Thermal We have defined work as force times distance and learned that work done on an object changes its kinetic. More simply put, heat energy, also called thermal energy or simply heat, is transferred from one location to another by particles bouncing into each other. Thermal energy is the energy due to the motion of atoms and molecules in a substance.. Heat Definition Thermal.
From thermalengineeringlearn.blogspot.com
THERMAL ENGINEERING Thermodynamic Equilibrium Heat Definition Thermal More simply put, heat energy, also called thermal energy or simply heat, is transferred from one location to another by particles bouncing into each other. We have defined work as force times distance and learned that work done on an object changes its kinetic. Thermal energy is the energy due to the motion of atoms and molecules in a substance.. Heat Definition Thermal.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID2977447 Heat Definition Thermal Heat, energy that is transferred from one body to another as the result of a difference in temperature. In thermodynamics, heat means energy which is. Define heat as transfer of energy. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Thermal energy. Heat Definition Thermal.
From becuo.com
Thermal Energy Examples And Definitions Images & Pictures Becuo Heat Definition Thermal In thermodynamics, heat means energy which is. We have defined work as force times distance and learned that work done on an object changes its kinetic. Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. This flow of heat continues until the two objects reach the same temperature. Define heat as transfer of energy.. Heat Definition Thermal.
From www.flinnsci.com
360 Science Thermal Energy and Heat Transfer Heat Definition Thermal Thermal energy is the energy due to the motion of atoms and molecules in a substance. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. We have defined work as force times distance and learned that work done on an object changes its kinetic. In thermodynamics, heat means energy which is. If two bodies at different temperatures. Heat Definition Thermal.
From www.youtube.com
What's the Difference between Heat and Temperature? YouTube Heat Definition Thermal We have defined work as force times distance and learned that work done on an object changes its kinetic. Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. This flow of heat continues until the two objects reach the same temperature. If two bodies at different temperatures are brought together, energy is. It accounts. Heat Definition Thermal.
From www.dreamstime.com
Thermal Energy Physics Definition, Example with Water and Heat Definition Thermal We have defined work as force times distance and learned that work done on an object changes its kinetic. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat, energy that is transferred from one body to another as the result of. Heat Definition Thermal.
From animalia-life.club
Examples Of Heat Energy Heat Definition Thermal It accounts for translational, vibrational, and rotational motion. This flow of heat continues until the two objects reach the same temperature. Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. As long as the temperature difference is maintained, a flow of heat. Heat Definition Thermal.
From animalia-life.club
Examples Of Heat Energy Heat Definition Thermal Heat, energy that is transferred from one body to another as the result of a difference in temperature. This flow of heat continues until the two objects reach the same temperature. Thermal energy is the energy due to the motion of atoms and molecules in a substance. All matter contains heat energy, and the more heat energy that is present,. Heat Definition Thermal.
From gamesmartz.com
Heat Easy to Understand Definition Heat Definition Thermal Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Thermal energy is the energy due to the motion of atoms and molecules in a substance. As long as the temperature difference is maintained, a flow of. Heat Definition Thermal.
From joipvxbwi.blob.core.windows.net
How Does Thermal Energy Transfer Hot To Cold at Charles Dion blog Heat Definition Thermal Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. It accounts for translational, vibrational, and rotational motion. All matter contains heat energy, and the more heat energy that is present, the hotter an item or area will be. This flow of heat continues until the two objects reach the same temperature. Heat is the. Heat Definition Thermal.
From www.slideserve.com
PPT Thermal Energy A. Temperature & Heat 1. Temperature is related to Heat Definition Thermal Thermal energy is the energy due to the motion of atoms and molecules in a substance. We have defined work as force times distance and learned that work done on an object changes its kinetic. In thermodynamics, heat means energy which is. Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. Heat, energy that. Heat Definition Thermal.
From www.slideserve.com
PPT Temperature, Thermal Energy, and Heat PowerPoint Presentation Heat Definition Thermal Define heat as transfer of energy. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. As long as the temperature difference is maintained, a flow of heat will occur. It accounts for translational, vibrational, and rotational motion. Thermal energy is the energy due to the motion of atoms and molecules in a substance. This. Heat Definition Thermal.
From www.slideserve.com
PPT THERMAL ENERGY & HEAT PowerPoint Presentation, free download ID Heat Definition Thermal Thermal energy is the energy due to the motion of atoms and molecules in a substance. More simply put, heat energy, also called thermal energy or simply heat, is transferred from one location to another by particles bouncing into each other. In thermodynamics, heat means energy which is. We have defined work as force times distance and learned that work. Heat Definition Thermal.
From study.com
Thermal Energy Definition & Examples Lesson Heat Definition Thermal It accounts for translational, vibrational, and rotational motion. This flow of heat continues until the two objects reach the same temperature. We have defined work as force times distance and learned that work done on an object changes its kinetic. In thermodynamics, heat means energy which is. Define heat as transfer of energy. More simply put, heat energy, also called. Heat Definition Thermal.
From www.slideshare.net
CHAPTER FIFTEEN Transmission of Heat Energy Heat Definition Thermal Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. All matter contains heat energy, and the more heat energy that is present, the hotter an item or area will be. Heat, energy that is transferred from one body to another as the result of a difference in temperature. We have defined work as force. Heat Definition Thermal.
From examples.yourdictionary.com
Difference Between Heat and Temperature in Simple Terms YourDictionary Heat Definition Thermal As long as the temperature difference is maintained, a flow of heat will occur. It accounts for translational, vibrational, and rotational motion. Thermal energy is the energy due to the motion of atoms and molecules in a substance. All matter contains heat energy, and the more heat energy that is present, the hotter an item or area will be. If. Heat Definition Thermal.
From arenahanna.wordpress.com
HEAT WORLD OF PHYSICS steps by steps to understand heat Heat Definition Thermal As long as the temperature difference is maintained, a flow of heat will occur. In thermodynamics, heat means energy which is. Define heat as transfer of energy. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Thermal energy is. Heat Definition Thermal.
From eduinput.com
Thermal EnergyDefinition, Types, And Examples Heat Definition Thermal In thermodynamics, heat means energy which is. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. As long as the temperature difference is maintained, a flow of heat will occur. Define heat as transfer of energy. If two bodies at different temperatures are brought together, energy is. More simply put, heat energy, also called thermal energy or. Heat Definition Thermal.
From sciencenotes.org
What Is Heat? Definition and Formulas Heat Definition Thermal More simply put, heat energy, also called thermal energy or simply heat, is transferred from one location to another by particles bouncing into each other. If two bodies at different temperatures are brought together, energy is. Thermal energy is the energy due to the motion of atoms and molecules in a substance. All matter contains heat energy, and the more. Heat Definition Thermal.
From www.yaclass.in
Thermal contact and Thermal Equilibrium — lesson. Science State Board Heat Definition Thermal Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Thermal energy is the energy due to the motion of atoms and molecules in a substance. In thermodynamics, heat means energy which is. It accounts for translational, vibrational, and rotational. Heat Definition Thermal.
From www.mechanicaleducation.com
Difference between Heat and Temperature Mechanical Education Heat Definition Thermal Thermal energy is the energy due to the motion of atoms and molecules in a substance. We have defined work as force times distance and learned that work done on an object changes its kinetic. Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. This flow of heat continues until the two objects reach. Heat Definition Thermal.
From fedinit.blogspot.com
Definition Of Heat And Thermal Energy FEDINIT Heat Definition Thermal It accounts for translational, vibrational, and rotational motion. Heat, energy that is transferred from one body to another as the result of a difference in temperature. More simply put, heat energy, also called thermal energy or simply heat, is transferred from one location to another by particles bouncing into each other. Define heat as transfer of energy. As long as. Heat Definition Thermal.
From study.com
Heat Energy Definition, Examples & Types Lesson Heat Definition Thermal All matter contains heat energy, and the more heat energy that is present, the hotter an item or area will be. If two bodies at different temperatures are brought together, energy is. This flow of heat continues until the two objects reach the same temperature. In thermodynamics, heat means energy which is. Define heat as transfer of energy. Thermal energy. Heat Definition Thermal.
From www.geeksforgeeks.org
What is Thermodynamics Definition, Laws, Formulas, Class 11 Notes Heat Definition Thermal Define heat as transfer of energy. As long as the temperature difference is maintained, a flow of heat will occur. All matter contains heat energy, and the more heat energy that is present, the hotter an item or area will be. This flow of heat continues until the two objects reach the same temperature. More simply put, heat energy, also. Heat Definition Thermal.
From www.slideserve.com
PPT Thermal Energy PowerPoint Presentation, free download ID5760611 Heat Definition Thermal We have defined work as force times distance and learned that work done on an object changes its kinetic. This flow of heat continues until the two objects reach the same temperature. Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat,. Heat Definition Thermal.
From www.worksheetsplanet.com
What is Thermal Energy Definition of Thermal Energy Heat Definition Thermal Heat, energy that is transferred from one body to another as the result of a difference in temperature. Define heat as transfer of energy. As long as the temperature difference is maintained, a flow of heat will occur. In thermodynamics, heat means energy which is. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. All matter contains. Heat Definition Thermal.
From www.sciencefacts.net
Thermal (Heat) Energy Definition, Examples, Equations, and Units Heat Definition Thermal If two bodies at different temperatures are brought together, energy is. Heat, energy that is transferred from one body to another as the result of a difference in temperature. This flow of heat continues until the two objects reach the same temperature. We have defined work as force times distance and learned that work done on an object changes its. Heat Definition Thermal.
From slideplayer.com
Chapter 6 Thermal Energy ppt download Heat Definition Thermal If two bodies at different temperatures are brought together, energy is. It accounts for translational, vibrational, and rotational motion. As long as the temperature difference is maintained, a flow of heat will occur. This flow of heat continues until the two objects reach the same temperature. More simply put, heat energy, also called thermal energy or simply heat, is transferred. Heat Definition Thermal.
From www.slideserve.com
PPT Physical Science Chapter 6 PowerPoint Presentation, free download Heat Definition Thermal More simply put, heat energy, also called thermal energy or simply heat, is transferred from one location to another by particles bouncing into each other. We have defined work as force times distance and learned that work done on an object changes its kinetic. This flow of heat continues until the two objects reach the same temperature. All matter contains. Heat Definition Thermal.
From www.thoughtco.com
Definition and Examples of Heat Energy Heat Definition Thermal We have defined work as force times distance and learned that work done on an object changes its kinetic. More simply put, heat energy, also called thermal energy or simply heat, is transferred from one location to another by particles bouncing into each other. Heat, energy that is transferred from one body to another as the result of a difference. Heat Definition Thermal.
From idronics.caleffi.com
4 THERMAL CHARACTERISTICS OF HEAT EXCHANGERS Caleffi Idronics Heat Definition Thermal In thermodynamics, heat means energy which is. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. This flow of heat continues until the two objects reach the same temperature. More simply put, heat energy, also called. Heat Definition Thermal.
From www.slideserve.com
PPT Chapter 6 Thermal Energy PowerPoint Presentation, free download Heat Definition Thermal Heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat, or thermal energy, is the sum of the kinetic energy of atoms or molecules. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Define heat as transfer of energy. In thermodynamics, heat means energy which is. All matter. Heat Definition Thermal.