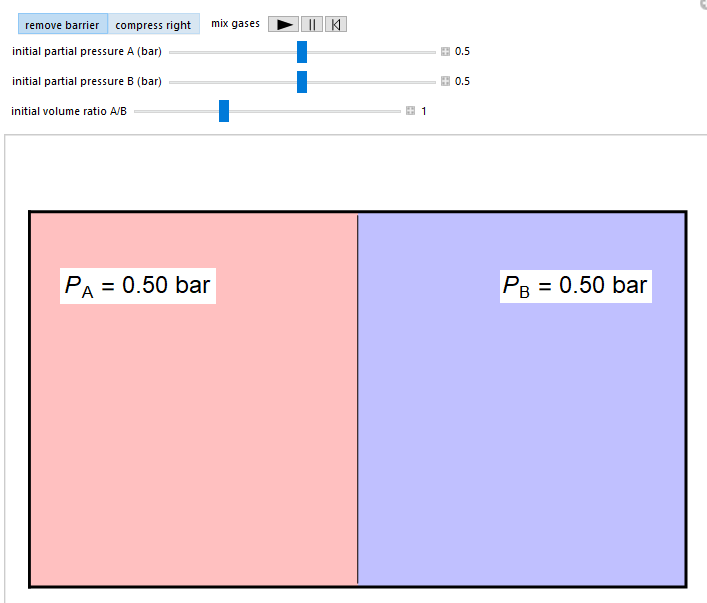
Ideal Gas For Entropy Change . The formulas for the entropy of an ideal gas. The heat transfer of a gas is equal to the heat capacity times the change in. Calculate the entropy change for 1.00 mol of an ideal gas expanding. P * v = r * t. For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described. Where r is the gas constant. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. For an ideal gas, the equation of state is written: Entropy change for a gas expansion. Entropy change in mixing of two ideal gases. Entropy changes in an ideal gas. Consider an insulated rigid container of gas separated into two halves by a heat conducting.
from learncheme.com
The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. Entropy changes in an ideal gas. For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described. The heat transfer of a gas is equal to the heat capacity times the change in. P * v = r * t. For an ideal gas, the equation of state is written: Entropy change in mixing of two ideal gases. Entropy change for a gas expansion. Calculate the entropy change for 1.00 mol of an ideal gas expanding. Where r is the gas constant.
entropychangesinmixingidealgases LearnChemE
Ideal Gas For Entropy Change Consider an insulated rigid container of gas separated into two halves by a heat conducting. Entropy change in mixing of two ideal gases. Entropy change for a gas expansion. Calculate the entropy change for 1.00 mol of an ideal gas expanding. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. Where r is the gas constant. P * v = r * t. For an ideal gas, the equation of state is written: The heat transfer of a gas is equal to the heat capacity times the change in. Consider an insulated rigid container of gas separated into two halves by a heat conducting. For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described. Entropy changes in an ideal gas. The formulas for the entropy of an ideal gas.
From physics.stackexchange.com
thermodynamics Entropy change of the ideal gas Physics Stack Exchange Ideal Gas For Entropy Change Calculate the entropy change for 1.00 mol of an ideal gas expanding. For an ideal gas, the equation of state is written: The heat transfer of a gas is equal to the heat capacity times the change in. For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy. Ideal Gas For Entropy Change.
From www.youtube.com
Entropy Change 2/3 Isothermal Irreversible Expansion of an Ideal Ideal Gas For Entropy Change For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described. Where r is the gas constant. Calculate the entropy change for 1.00 mol of an ideal gas expanding. Entropy changes in an ideal gas. The fact that we can derive it from statistical mechanics is evidence. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Entropy Change PowerPoint Presentation, free download ID3432482 Ideal Gas For Entropy Change Entropy change for a gas expansion. Entropy change in mixing of two ideal gases. The formulas for the entropy of an ideal gas. Consider an insulated rigid container of gas separated into two halves by a heat conducting. The heat transfer of a gas is equal to the heat capacity times the change in. Where r is the gas constant.. Ideal Gas For Entropy Change.
From www.numerade.com
SOLVEDA system with 1 mole of an ideal gas changes its volume from 10 Ideal Gas For Entropy Change The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. The formulas for the entropy of an ideal gas. Where r is the gas constant. Calculate the entropy change for 1.00 mol of an ideal gas expanding. For an ideal gas, the equation of state is written: P. Ideal Gas For Entropy Change.
From www.youtube.com
ideal gas mixture change in entropy calculation YouTube Ideal Gas For Entropy Change The heat transfer of a gas is equal to the heat capacity times the change in. Entropy change in mixing of two ideal gases. The formulas for the entropy of an ideal gas. For an ideal gas, the equation of state is written: The fact that we can derive it from statistical mechanics is evidence in favor of our quantum. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Calculating Entropy Change PowerPoint Presentation, free download Ideal Gas For Entropy Change Where r is the gas constant. For an ideal gas, the equation of state is written: The formulas for the entropy of an ideal gas. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. Calculate the entropy change for 1.00 mol of an ideal gas expanding. For. Ideal Gas For Entropy Change.
From www.numerade.com
SOLVED A monoatomic ideal gas (n moles) goes through a cyclic process Ideal Gas For Entropy Change Where r is the gas constant. Calculate the entropy change for 1.00 mol of an ideal gas expanding. The heat transfer of a gas is equal to the heat capacity times the change in. Entropy change in mixing of two ideal gases. Consider an insulated rigid container of gas separated into two halves by a heat conducting. Entropy changes in. Ideal Gas For Entropy Change.
From www.youtube.com
Enthalpy Change with Pressure for an Ideal Gas at Constant Entropy Ideal Gas For Entropy Change The heat transfer of a gas is equal to the heat capacity times the change in. P * v = r * t. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. Calculate the entropy change for 1.00 mol of an ideal gas expanding. For an ideal. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Calculating Entropy Change PowerPoint Presentation, free download Ideal Gas For Entropy Change Entropy changes in an ideal gas. The heat transfer of a gas is equal to the heat capacity times the change in. Where r is the gas constant. Consider an insulated rigid container of gas separated into two halves by a heat conducting. Entropy change for a gas expansion. For an ideal gas, the equation of state is written: The. Ideal Gas For Entropy Change.
From www.youtube.com
Entropy Change for an ideal gas Thermodynamics Physical Chemistry Ideal Gas For Entropy Change Where r is the gas constant. The formulas for the entropy of an ideal gas. Entropy changes in an ideal gas. Entropy change for a gas expansion. Consider an insulated rigid container of gas separated into two halves by a heat conducting. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical. Ideal Gas For Entropy Change.
From www.numerade.com
SOLVED Consider a process in which an ideal gas is compressed to one Ideal Gas For Entropy Change The formulas for the entropy of an ideal gas. Entropy change for a gas expansion. For an ideal gas, the equation of state is written: For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described. Entropy change in mixing of two ideal gases. Where r is. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID2494031 Ideal Gas For Entropy Change Entropy changes in an ideal gas. Calculate the entropy change for 1.00 mol of an ideal gas expanding. Consider an insulated rigid container of gas separated into two halves by a heat conducting. Where r is the gas constant. P * v = r * t. For an ideal gas, the equation of state is written: Entropy change for a. Ideal Gas For Entropy Change.
From www.youtube.com
How To Calculate Entropy Changes Ideal Gases YouTube Ideal Gas For Entropy Change The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described. For an ideal gas, the equation of state is written: Entropy change for a. Ideal Gas For Entropy Change.
From www.researchgate.net
Comparison of entropy and enthalpy changes for realgas and idealgas Ideal Gas For Entropy Change Where r is the gas constant. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. The heat transfer of a gas is equal to the heat capacity times the change in. The formulas for the entropy of an ideal gas. For an ideal gas traversing a carnot. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Entropy, probability and disorder PowerPoint Presentation, free Ideal Gas For Entropy Change The formulas for the entropy of an ideal gas. Consider an insulated rigid container of gas separated into two halves by a heat conducting. Entropy changes in an ideal gas. Where r is the gas constant. For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described.. Ideal Gas For Entropy Change.
From www.youtube.com
Entropy Change for Ideal Gas Expansion YouTube Ideal Gas For Entropy Change The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. The heat transfer of a gas is equal to the heat capacity times the change in. Where r is the gas constant. Calculate the entropy change for 1.00 mol of an ideal gas expanding. Entropy change in mixing. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT The Second Law of Thermodynamics PowerPoint Presentation, free Ideal Gas For Entropy Change Entropy changes in an ideal gas. For an ideal gas, the equation of state is written: P * v = r * t. Entropy change for a gas expansion. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. Entropy change in mixing of two ideal gases. For. Ideal Gas For Entropy Change.
From www.youtube.com
entropy change for an ideal gas entropy of ideal gas derivation Ideal Gas For Entropy Change Consider an insulated rigid container of gas separated into two halves by a heat conducting. P * v = r * t. Where r is the gas constant. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. For an ideal gas traversing a carnot cycle, we have. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT The TdS Equations & Diagrams PowerPoint Presentation, free Ideal Gas For Entropy Change For an ideal gas, the equation of state is written: Entropy change in mixing of two ideal gases. Where r is the gas constant. Calculate the entropy change for 1.00 mol of an ideal gas expanding. Consider an insulated rigid container of gas separated into two halves by a heat conducting. P * v = r * t. The fact. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT The TdS Equations & Diagrams PowerPoint Presentation, free Ideal Gas For Entropy Change Where r is the gas constant. Calculate the entropy change for 1.00 mol of an ideal gas expanding. Entropy change in mixing of two ideal gases. Entropy changes in an ideal gas. Consider an insulated rigid container of gas separated into two halves by a heat conducting. For an ideal gas, the equation of state is written: The heat transfer. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Calculating Entropy Change PowerPoint Presentation, free download Ideal Gas For Entropy Change Entropy change for a gas expansion. Where r is the gas constant. The heat transfer of a gas is equal to the heat capacity times the change in. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. For an ideal gas, the equation of state is written:. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Calculating Entropy Change PowerPoint Presentation, free download Ideal Gas For Entropy Change The formulas for the entropy of an ideal gas. The heat transfer of a gas is equal to the heat capacity times the change in. Calculate the entropy change for 1.00 mol of an ideal gas expanding. Entropy change in mixing of two ideal gases. P * v = r * t. For an ideal gas, the equation of state. Ideal Gas For Entropy Change.
From learncheme.com
entropychangesinmixingidealgases LearnChemE Ideal Gas For Entropy Change Calculate the entropy change for 1.00 mol of an ideal gas expanding. P * v = r * t. Entropy changes in an ideal gas. Where r is the gas constant. For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described. Entropy change in mixing of. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT ENGR 2213 Thermodynamics PowerPoint Presentation, free download Ideal Gas For Entropy Change The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. Entropy change in mixing of two ideal gases. The heat transfer of a gas is equal to the heat capacity times the change in. Where r is the gas constant. P * v = r * t. The. Ideal Gas For Entropy Change.
From www.youtube.com
Calculating change in Entropy of an ideal gas Thermodynamics YouTube Ideal Gas For Entropy Change Entropy changes in an ideal gas. Entropy change for a gas expansion. Where r is the gas constant. Calculate the entropy change for 1.00 mol of an ideal gas expanding. The formulas for the entropy of an ideal gas. Entropy change in mixing of two ideal gases. For an ideal gas traversing a carnot cycle, we have shown that \[\delta. Ideal Gas For Entropy Change.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 8, pt 5 of 5 Entropy of Ideal Gas For Entropy Change For an ideal gas, the equation of state is written: Calculate the entropy change for 1.00 mol of an ideal gas expanding. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. Entropy change for a gas expansion. P * v = r * t. Where r is. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Entropy PowerPoint Presentation, free download ID6765953 Ideal Gas For Entropy Change The formulas for the entropy of an ideal gas. For an ideal gas, the equation of state is written: Calculate the entropy change for 1.00 mol of an ideal gas expanding. Entropy change in mixing of two ideal gases. P * v = r * t. The fact that we can derive it from statistical mechanics is evidence in favor. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT The TdS Equations & Diagrams PowerPoint Presentation, free Ideal Gas For Entropy Change Calculate the entropy change for 1.00 mol of an ideal gas expanding. Where r is the gas constant. For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described. For an ideal gas, the equation of state is written: The heat transfer of a gas is equal. Ideal Gas For Entropy Change.
From www.grc.nasa.gov
Entropy of a Gas Ideal Gas For Entropy Change The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described. For an ideal gas, the equation of state is written: Calculate the entropy change. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Calculating Entropy Change PowerPoint Presentation, free download Ideal Gas For Entropy Change P * v = r * t. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. The formulas for the entropy of an ideal gas. For an ideal gas, the equation of state is written: Entropy change for a gas expansion. The heat transfer of a gas. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Entropy Change PowerPoint Presentation, free download ID6772170 Ideal Gas For Entropy Change For an ideal gas, the equation of state is written: Consider an insulated rigid container of gas separated into two halves by a heat conducting. Entropy changes in an ideal gas. For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described. Where r is the gas. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Calculating Entropy Change PowerPoint Presentation, free download Ideal Gas For Entropy Change For an ideal gas traversing a carnot cycle, we have shown that \[\delta s=\oint{ds}=\oint{\frac{dq^{rev}}{t}}=0 \nonumber \] \(s\) is, of course, the entropy function described. The formulas for the entropy of an ideal gas. Calculate the entropy change for 1.00 mol of an ideal gas expanding. Consider an insulated rigid container of gas separated into two halves by a heat conducting.. Ideal Gas For Entropy Change.
From www.chegg.com
Solved Show that the change of entropy of an ideal gas which Ideal Gas For Entropy Change The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. Entropy changes in an ideal gas. Entropy change for a gas expansion. For an ideal gas, the equation of state is written: Calculate the entropy change for 1.00 mol of an ideal gas expanding. Consider an insulated rigid. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Calculating Entropy Change PowerPoint Presentation, free download Ideal Gas For Entropy Change Entropy change for a gas expansion. Entropy change in mixing of two ideal gases. Calculate the entropy change for 1.00 mol of an ideal gas expanding. P * v = r * t. Entropy changes in an ideal gas. Where r is the gas constant. For an ideal gas, the equation of state is written: Consider an insulated rigid container. Ideal Gas For Entropy Change.
From www.slideserve.com
PPT Calculating Entropy Change PowerPoint Presentation, free download Ideal Gas For Entropy Change For an ideal gas, the equation of state is written: The heat transfer of a gas is equal to the heat capacity times the change in. The fact that we can derive it from statistical mechanics is evidence in favor of our quantum mechanical model of a gas. P * v = r * t. The formulas for the entropy. Ideal Gas For Entropy Change.