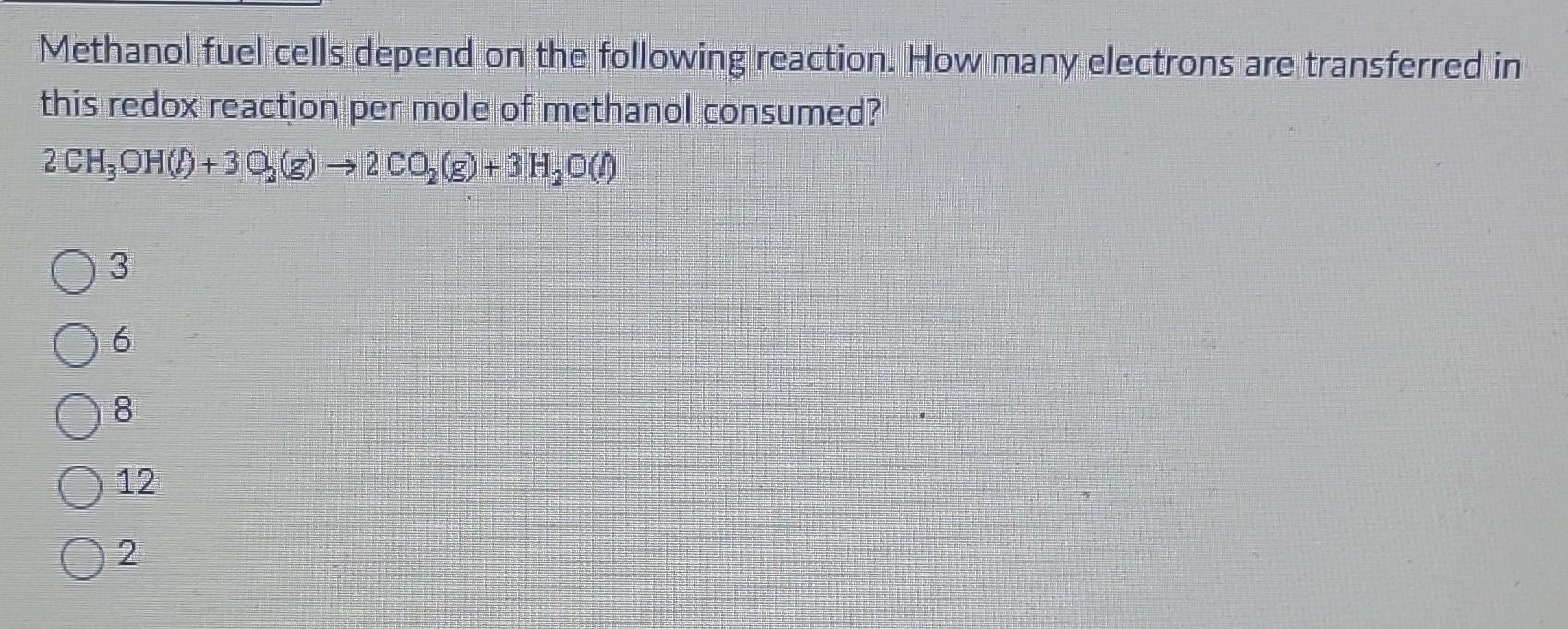Methanol Fuel Cells Use The Following Reaction . 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. What is the coefficient for. How many electrons are transferred in this redox reaction as written? In o2, the oxidation number of o is 0. How many electrons are transferred in this redox reaction as written? Identify the unbalanced chemical reaction. Methanol fuel cells use the following reaction. Methanol fuel cells use the following reaction. The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. Balance the redox reaction by the oxidation number method. Methanol using a fuel cell. In the oxidation half of this redox reaction,. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel cell, and the. 2ch3oh + 3o2 → 2co2 + 3h2o a. The reaction in a methanol fuel cell is between methanol (ch₃oh) and oxygen.
from www.chegg.com
2ch3oh + 3o2 → 2co2 + 3h2o a. In methanol fuel cells, the methanol (ch3oh) is oxidized, and the oxygen (o2) is reduced. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated. Methanol using a fuel cell. The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. In o2, the oxidation number of o is 0. The reaction in a methanol fuel cell is between methanol (ch₃oh) and oxygen. Methanol fuel cells use the following reaction. Balance the redox reaction by the oxidation number method.
Solved Methanol fuel cells depend on the following reaction.
Methanol Fuel Cells Use The Following Reaction Methanol fuel cells use the following reaction. The reaction in a methanol fuel cell is between methanol (ch₃oh) and oxygen. Methanol fuel cells use the following reaction. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated. Methanol using a fuel cell. 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel cell, and the. Balance the redox reaction by the oxidation number method. How many electrons are transferred in this redox reaction as written? In the oxidation half of this redox reaction,. Identify the unbalanced chemical reaction. In o2, the oxidation number of o is 0. What is the coefficient for. In methanol fuel cells, the methanol (ch3oh) is oxidized, and the oxygen (o2) is reduced. How many electrons are transferred in this redox reaction as written? 2ch3oh + 3o2 → 2co2 + 3h2o a.
From us.metoree.com
9 Methanol Fuel Cell Manufacturers in 2024 Metoree Methanol Fuel Cells Use The Following Reaction 2ch3oh + 3o2 → 2co2 + 3h2o a. Balance the redox reaction by the oxidation number method. What is the coefficient for. Identify the unbalanced chemical reaction. How many electrons are transferred in this redox reaction as written? Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process. Methanol Fuel Cells Use The Following Reaction.
From www.chegg.com
The total reaction of a direct methanol fuel cell Methanol Fuel Cells Use The Following Reaction How many electrons are transferred in this redox reaction as written? In the oxidation half of this redox reaction,. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated. In methanol fuel cells, the methanol (ch3oh) is oxidized, and the oxygen (o2) is. Methanol Fuel Cells Use The Following Reaction.
From www.chegg.com
Solved Methanol fuel cells depend on the following reaction. Methanol Fuel Cells Use The Following Reaction Methanol using a fuel cell. In the oxidation half of this redox reaction,. Methanol fuel cells use the following reaction. What is the coefficient for. The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. In o2, the oxidation number of o is 0. In methanol fuel cells, the. Methanol Fuel Cells Use The Following Reaction.
From solvedlib.com
The reaction taking place in a methanol fuel cell is… SolvedLib Methanol Fuel Cells Use The Following Reaction Balance the redox reaction by the oxidation number method. Methanol fuel cells use the following reaction. The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. How many electrons are transferred in this redox reaction as written? In the oxidation half of this redox reaction,. Identify the unbalanced chemical. Methanol Fuel Cells Use The Following Reaction.
From www.researchgate.net
Chemical reactions of direct methanol fuel cell (DMFC) Download Methanol Fuel Cells Use The Following Reaction Methanol fuel cells use the following reaction. The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. Identify the unbalanced chemical reaction. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated.. Methanol Fuel Cells Use The Following Reaction.
From www.researchgate.net
3 Methanol Fuel Cell Power Characterization Circuitry Download Methanol Fuel Cells Use The Following Reaction By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel cell, and the. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated. Methanol fuel. Methanol Fuel Cells Use The Following Reaction.
From www.slideserve.com
PPT DIRECT METHANOL FUEL CELL WITH EXTENDED REACTION ZONE ANODE Methanol Fuel Cells Use The Following Reaction How many electrons are transferred in this redox reaction as written? Balance the redox reaction by the oxidation number method. Identify the unbalanced chemical reaction. The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. In the oxidation half of this redox reaction,. By contrast to a conventional cell,. Methanol Fuel Cells Use The Following Reaction.
From chempedia.info
Direct methanol fuel cell schematic diagram Big Chemical Encyclopedia Methanol Fuel Cells Use The Following Reaction 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. Identify the unbalanced chemical reaction. Methanol fuel cells use the following reaction. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated. What is the coefficient for. Methanol fuel cells use the following reaction. In o2,. Methanol Fuel Cells Use The Following Reaction.
From www.slideserve.com
PPT Direct Methanol Fuel Cell Study on anode and cathode catalysts Methanol Fuel Cells Use The Following Reaction By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel cell, and the. Methanol fuel cells use the following reaction. Balance the redox reaction by the oxidation number method. How many electrons are transferred in this redox reaction as written? How many. Methanol Fuel Cells Use The Following Reaction.
From www.numerade.com
SOLVED Consider a direct methanol fuel cell with a liquid methanol and Methanol Fuel Cells Use The Following Reaction How many electrons are transferred in this redox reaction as written? In o2, the oxidation number of o is 0. Methanol fuel cells use the following reaction. What is the coefficient for. The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. In methanol fuel cells, the methanol (ch3oh). Methanol Fuel Cells Use The Following Reaction.
From www.myelectrical2015.com
Electrical Revolution Methanol Fuel Cells Use The Following Reaction How many electrons are transferred in this redox reaction as written? Methanol fuel cells use the following reaction. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel cell, and the. 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. In the oxidation half. Methanol Fuel Cells Use The Following Reaction.
From www.researchgate.net
Oxidation reactions in fuel cells (cathodic and anodic half reactions Methanol Fuel Cells Use The Following Reaction 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. Methanol fuel cells use the following reaction. The reaction in a methanol fuel cell is between methanol (ch₃oh) and oxygen. 2ch3oh + 3o2 → 2co2 + 3h2o a. In methanol fuel cells,. Methanol Fuel Cells Use The Following Reaction.
From siqens.de
Methanol fuel cell Working principle and different types SIQENS Methanol Fuel Cells Use The Following Reaction Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel cell, and the. Methanol fuel. Methanol Fuel Cells Use The Following Reaction.
From www.researchgate.net
a Schematically showing the methanol fuel cells. b The exploded view of Methanol Fuel Cells Use The Following Reaction Identify the unbalanced chemical reaction. How many electrons are transferred in this redox reaction as written? In methanol fuel cells, the methanol (ch3oh) is oxidized, and the oxygen (o2) is reduced. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated. What is. Methanol Fuel Cells Use The Following Reaction.
From www.chegg.com
Solved Direct methanol fuel cells (DMFCs) have shown some Methanol Fuel Cells Use The Following Reaction 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. In o2, the oxidation number of o is 0. In the oxidation half of this redox reaction,. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated. The reaction in a methanol fuel cell is between. Methanol Fuel Cells Use The Following Reaction.
From www.numerade.com
SOLVED Use the following information to answer numericalresponse Methanol Fuel Cells Use The Following Reaction The reaction in a methanol fuel cell is between methanol (ch₃oh) and oxygen. Methanol fuel cells use the following reaction. 2ch3oh + 3o2 → 2co2 + 3h2o a. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel cell, and the. The. Methanol Fuel Cells Use The Following Reaction.
From www.numerade.com
SOLVED In a direct methanol fuel cell, methanol is used as a fuel Methanol Fuel Cells Use The Following Reaction How many electrons are transferred in this redox reaction as written? Methanol fuel cells use the following reaction. In o2, the oxidation number of o is 0. Identify the unbalanced chemical reaction. 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the. Methanol Fuel Cells Use The Following Reaction.
From www.chegg.com
Solved A "direct methanol fuel cell" uses chemical reactions Methanol Fuel Cells Use The Following Reaction 2ch3oh + 3o2 → 2co2 + 3h2o a. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel cell, and the. Balance the redox reaction by the oxidation number method. In the oxidation half of this redox reaction,. In o2, the oxidation. Methanol Fuel Cells Use The Following Reaction.
From siqens.de
Methanol fuel cell Working principle and different types SIQENS Methanol Fuel Cells Use The Following Reaction 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. In the oxidation half of this redox reaction,. Balance the redox reaction by the oxidation number method. Methanol fuel cells use the following reaction. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel cell,. Methanol Fuel Cells Use The Following Reaction.
From www.chegg.com
Solved The total reaction of a direct methanol fuel cell Methanol Fuel Cells Use The Following Reaction In the oxidation half of this redox reaction,. Methanol using a fuel cell. 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. How many electrons are transferred in this redox reaction as written? 2ch3oh + 3o2 → 2co2 + 3h2o a. Identify the unbalanced chemical reaction. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent. Methanol Fuel Cells Use The Following Reaction.
From www.chegg.com
Solved DMFC fuel crossover. In the direct methanol fuel cell Methanol Fuel Cells Use The Following Reaction Identify the unbalanced chemical reaction. Methanol using a fuel cell. The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. In methanol fuel cells, the methanol (ch3oh) is oxidized, and the oxygen (o2) is reduced. Methanol fuel cells use the following reaction. How many electrons are transferred in this. Methanol Fuel Cells Use The Following Reaction.
From www.numerade.com
SOLVED Methanol fuel cells depend on the following reaction. How many Methanol Fuel Cells Use The Following Reaction Methanol fuel cells use the following reaction. How many electrons are transferred in this redox reaction as written? How many electrons are transferred in this redox reaction as written? 2ch3oh + 3o2 → 2co2 + 3h2o a. The reaction in a methanol fuel cell is between methanol (ch₃oh) and oxygen. Balance the redox reaction by the oxidation number method. What. Methanol Fuel Cells Use The Following Reaction.
From handwiki.org
ChemistryReformed methanol fuel cell HandWiki Methanol Fuel Cells Use The Following Reaction Balance the redox reaction by the oxidation number method. What is the coefficient for. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a. Methanol Fuel Cells Use The Following Reaction.
From siqens.de
Methanol fuel cell Working principle and different types SIQENS Methanol Fuel Cells Use The Following Reaction Identify the unbalanced chemical reaction. 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. What is the coefficient for. The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. Balance the redox reaction by the oxidation number method. How many electrons are transferred in this redox reaction as written? In methanol. Methanol Fuel Cells Use The Following Reaction.
From www.researchgate.net
Fuel cell reactions are depicted for a) methanol and b) glycerol using Methanol Fuel Cells Use The Following Reaction In o2, the oxidation number of o is 0. How many electrons are transferred in this redox reaction as written? Methanol fuel cells use the following reaction. Identify the unbalanced chemical reaction. What is the coefficient for. In methanol fuel cells, the methanol (ch3oh) is oxidized, and the oxygen (o2) is reduced. Balance the redox reaction by the oxidation number. Methanol Fuel Cells Use The Following Reaction.
From www.researchgate.net
The schematic diagram of the carbonneutralized direct methanol fuel Methanol Fuel Cells Use The Following Reaction The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. In o2, the oxidation number of o is 0. What is the coefficient for. How many electrons are transferred in this redox reaction as written? Identify the unbalanced chemical reaction. Balance the redox reaction by the oxidation number method.. Methanol Fuel Cells Use The Following Reaction.
From www.researchgate.net
Schematic representation of Direct Methanol Fuel Cell (DMFC). This fuel Methanol Fuel Cells Use The Following Reaction By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel cell, and the. In the oxidation half of this redox reaction,. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the. Methanol Fuel Cells Use The Following Reaction.
From www.researchgate.net
Schematic illustration of a direct methanol fuel cell (DMFC Methanol Fuel Cells Use The Following Reaction Methanol fuel cells use the following reaction. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated. Balance the redox reaction by the oxidation number method. What is the coefficient for. In methanol fuel cells, the methanol (ch3oh) is oxidized, and the oxygen. Methanol Fuel Cells Use The Following Reaction.
From www.coursehero.com
[Solved] 1. Methanol fuel cells use the following reaction. How many Methanol Fuel Cells Use The Following Reaction The reaction in a methanol fuel cell is between methanol (ch₃oh) and oxygen. How many electrons are transferred in this redox reaction as written? By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel cell, and the. 2ch3oh + 3o2 âž¡ï¸ 2co2. Methanol Fuel Cells Use The Following Reaction.
From www.youtube.com
Direct Methanol Fuel Cell, Introduction, Principle, Advantages Methanol Fuel Cells Use The Following Reaction 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. Balance the redox reaction by the oxidation number method. Normally, when you run a methanol fuel cell, methanol and oxygen react to make water and carbon dioxide, and in the process an electrical current is generated. Methanol using a fuel cell. By contrast to a conventional cell, where only limited quantities of oxidizing. Methanol Fuel Cells Use The Following Reaction.
From www.researchgate.net
Schematic representation of Direct Methanol Fuel Cell (DMFC). This fuel Methanol Fuel Cells Use The Following Reaction Methanol using a fuel cell. 2ch3oh + 3o2 → 2co2 + 3h2o a. How many electrons are transferred in this redox reaction as written? The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. Methanol fuel cells use the following reaction. Methanol fuel cells use the following reaction. In. Methanol Fuel Cells Use The Following Reaction.
From www.slideserve.com
PPT Development of Direct Methanol Fuel Cell and Special Proton Methanol Fuel Cells Use The Following Reaction The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. Methanol fuel cells use the following reaction. How many electrons are transferred in this redox reaction as written? In the oxidation half of this redox reaction,. What is the coefficient for. Methanol using a fuel cell. How many electrons. Methanol Fuel Cells Use The Following Reaction.
From www.chegg.com
Solved Methanol fuel cells produce electricity by reacting Methanol Fuel Cells Use The Following Reaction Identify the unbalanced chemical reaction. 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. How many electrons are transferred in this redox reaction as written? Balance the redox reaction by the oxidation number method. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to a fuel. Methanol Fuel Cells Use The Following Reaction.
From www.researchgate.net
Linear sweep voltammetry for the methanol oxidation reaction in 0.1 M Methanol Fuel Cells Use The Following Reaction The reaction in a methanol fuel cell is between methanol (ch₃oh) and oxygen. The methanol (ch3oh) molecule loses 6 electrons and gets oxidized to form co2, while the oxygen (o2) molecule gains 4 electrons. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing agent are available, a continuous supply of both is provided to. Methanol Fuel Cells Use The Following Reaction.
From www.youtube.com
Methanol Oxygen Fuel Cells Part 3 Dr Anima Upadhyay YouTube Methanol Fuel Cells Use The Following Reaction Methanol fuel cells use the following reaction. Identify the unbalanced chemical reaction. 2ch3oh + 3o2 âž¡ï¸ 2co2 + 3h2o. In o2, the oxidation number of o is 0. In the oxidation half of this redox reaction,. How many electrons are transferred in this redox reaction as written? By contrast to a conventional cell, where only limited quantities of oxidizing agent. Methanol Fuel Cells Use The Following Reaction.