Catalyst Kinetics Chemistry . Learn about different types of catalysts in chemistry and their role in chemical reactions. In this lecture, catalysts of different. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Instead they provide a new mechanism for a reaction to. This file contains information regarding lecture 34. Catalysts allow a reaction to. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant.
from slidetodoc.com
They do not appear in the reaction’s net equation and are not consumed during the reaction. In this lecture, catalysts of different. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts participate in a chemical reaction and increase its rate. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. This file contains information regarding lecture 34. Catalysts allow a reaction to. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Instead they provide a new mechanism for a reaction to.
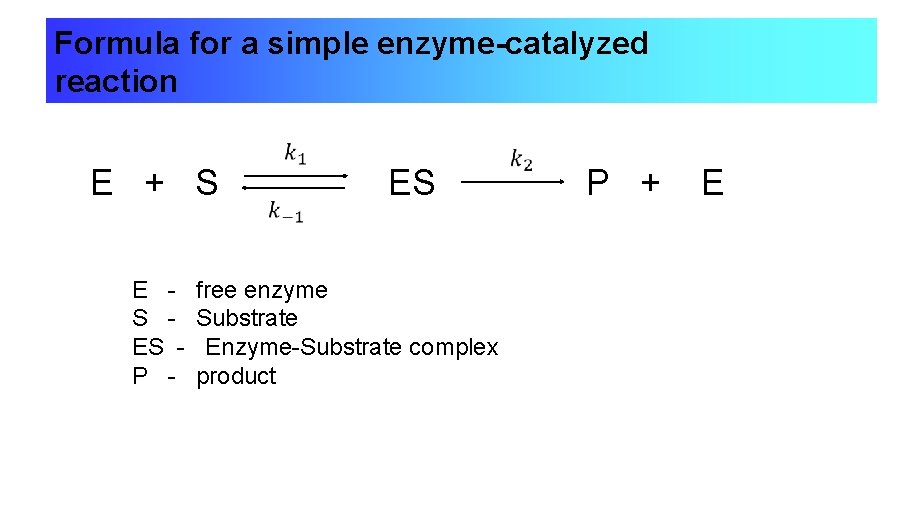
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS
Catalyst Kinetics Chemistry This file contains information regarding lecture 34. They do not appear in the reaction’s net equation and are not consumed during the reaction. Learn about different types of catalysts in chemistry and their role in chemical reactions. Instead they provide a new mechanism for a reaction to. Catalysts allow a reaction to. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts participate in a chemical reaction and increase its rate. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. This file contains information regarding lecture 34. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. In this lecture, catalysts of different.
From ppt-online.org
Rates of reaction презентация онлайн Catalyst Kinetics Chemistry Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. This file contains information regarding lecture 34. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed. Catalyst Kinetics Chemistry.
From rumble.com
Surface Catalysis, Catalyst Types, Chemistry Catalyst Kinetics Chemistry Catalysts participate in a chemical reaction and increase its rate. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant. This file contains information regarding lecture 34. They do not appear in the reaction’s net equation and are not consumed during the reaction. Learn about different types of catalysts in chemistry and their role in chemical. Catalyst Kinetics Chemistry.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Kinetics Chemistry Catalysts participate in a chemical reaction and increase its rate. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts lower. Catalyst Kinetics Chemistry.
From www.differencebetween.com
What is the Difference Between Chemical and Chemical Catalyst Kinetics Chemistry Catalysts allow a reaction to. In this lecture, catalysts of different. Catalysts participate in a chemical reaction and increase its rate. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Instead they provide a new mechanism for a. Catalyst Kinetics Chemistry.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Kinetics Chemistry Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. In this lecture, catalysts of different. This file contains information regarding lecture 34. Catalysts lower. Catalyst Kinetics Chemistry.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Kinetics Chemistry Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. Instead they provide a new mechanism for a reaction to. Catalysts can lower the activation. Catalyst Kinetics Chemistry.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID4503154 Catalyst Kinetics Chemistry Instead they provide a new mechanism for a reaction to. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. In this lecture, catalysts of. Catalyst Kinetics Chemistry.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID Catalyst Kinetics Chemistry In this lecture, catalysts of different. Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. They do not. Catalyst Kinetics Chemistry.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID5829521 Catalyst Kinetics Chemistry Instead they provide a new mechanism for a reaction to. In this lecture, catalysts of different. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts lower the activation energy barrier for a reaction without changing. Catalyst Kinetics Chemistry.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free Catalyst Kinetics Chemistry Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts allow a reaction to. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. In this lecture, catalysts of different. Catalysts lower the activation. Catalyst Kinetics Chemistry.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalyst Kinetics Chemistry Instead they provide a new mechanism for a reaction to. In this lecture, catalysts of different. Learn about different types of catalysts in chemistry and their role in chemical reactions. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts are defined as substances that participate in a chemical reaction but are not. Catalyst Kinetics Chemistry.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Kinetics Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to. In this lecture, catalysts of different. This file contains information. Catalyst Kinetics Chemistry.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Catalyst Kinetics Chemistry In this lecture, catalysts of different. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Learn about different types of catalysts in chemistry and their role in chemical reactions. Instead they provide a. Catalyst Kinetics Chemistry.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Catalyst Kinetics Chemistry Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. This file contains information regarding lecture 34. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts allow a reaction to. Catalysts participate in a chemical reaction and increase its rate. Instead they provide a new mechanism for. Catalyst Kinetics Chemistry.
From www.britannica.com
Activation energy Definition & Facts Britannica Catalyst Kinetics Chemistry Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. In this lecture, catalysts of different. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Instead. Catalyst Kinetics Chemistry.
From www.studocu.com
Chemical and Catalysis CHEMICAL 1 Chemical Catalyst Kinetics Chemistry Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts allow a reaction to. Learn about different types of catalysts in chemistry and their role in chemical reactions. Instead they provide a new mechanism for a reaction to. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant.. Catalyst Kinetics Chemistry.
From www.youtube.com
19 Chemical Effect of Catalyst on Rate of Reaction IIT Catalyst Kinetics Chemistry Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. This file contains information regarding lecture 34. Catalysts allow a reaction to. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. Learn about different types of. Catalyst Kinetics Chemistry.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Kinetics Chemistry Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. This file contains information regarding lecture 34. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts allow a. Catalyst Kinetics Chemistry.
From www.savemyexams.co.uk
Catalysts (1.7.6) AQA A Level Chemistry Revision Notes 2017 Save My Catalyst Kinetics Chemistry Catalysts allow a reaction to. Learn about different types of catalysts in chemistry and their role in chemical reactions. In this lecture, catalysts of different. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant. This file. Catalyst Kinetics Chemistry.
From rumble.com
Enzyme Catalysis, Catalyst Types, Chemistry Catalyst Kinetics Chemistry Learn about different types of catalysts in chemistry and their role in chemical reactions. Instead they provide a new mechanism for a reaction to. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Catalysts allow a reaction to. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant.. Catalyst Kinetics Chemistry.
From www.youtube.com
Effect of Catalyst on Rate of Reaction Chemical Chemistry Catalyst Kinetics Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts participate in a chemical reaction and increase its rate. Catalysts can lower the activation energy. Catalyst Kinetics Chemistry.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Kinetics Chemistry Instead they provide a new mechanism for a reaction to. This file contains information regarding lecture 34. Catalysts allow a reaction to. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. They do not appear. Catalyst Kinetics Chemistry.
From www.youtube.com
& CATALYSIS ACID BASE CATALYSIS ONLINE CHEMISTRY YouTube Catalyst Kinetics Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. Instead they provide a new mechanism for a reaction to. Catalysts participate in a chemical reaction and increase its rate. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Learn about different types of catalysts in. Catalyst Kinetics Chemistry.
From 2012books.lardbucket.org
Catalysis Catalyst Kinetics Chemistry Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts allow a reaction to proceed via a pathway that has a. Catalyst Kinetics Chemistry.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalyst Kinetics Chemistry This file contains information regarding lecture 34. Learn about different types of catalysts in chemistry and their role in chemical reactions. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Instead they provide a new mechanism for a reaction to. Catalysts can lower the activation energy required for reactions, allowing them to. Catalyst Kinetics Chemistry.
From rumble.com
AcidBase Catalysis, Catalyst Types, Chemistry Catalyst Kinetics Chemistry Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Learn about different types of catalysts in chemistry and their role in chemical reactions. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium. Catalyst Kinetics Chemistry.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Kinetics Chemistry Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. In this lecture, catalysts of different. Instead they provide a new mechanism. Catalyst Kinetics Chemistry.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID5829521 Catalyst Kinetics Chemistry Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Instead they provide a new mechanism for a reaction to. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Catalysts participate in a chemical reaction and increase its rate. Catalysts lower the activation energy barrier. Catalyst Kinetics Chemistry.
From www.slideserve.com
PPT Chemical Part 3 Reaction Mechanisms PowerPoint Catalyst Kinetics Chemistry Catalysts allow a reaction to. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. In this lecture, catalysts of different. Instead they provide a new mechanism for a reaction to. They do not appear in the reaction’s net equation and are not consumed during the reaction. Learn about different types of catalysts. Catalyst Kinetics Chemistry.
From www.youtube.com
CHEMICAL &CATALYSIS YouTube Catalyst Kinetics Chemistry Catalysts participate in a chemical reaction and increase its rate. In this lecture, catalysts of different. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Instead they provide a new mechanism for a reaction to.. Catalyst Kinetics Chemistry.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Kinetics Chemistry Catalysts allow a reaction to. Catalysts lower the activation energy barrier for a reaction without changing the equilibrium constant. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. This file contains information regarding lecture 34. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently.. Catalyst Kinetics Chemistry.
From www.youtube.com
A catalyst CLASS 12 CHEMICAL CHEMISTRY Doubtnut YouTube Catalyst Kinetics Chemistry Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Learn about different types of catalysts in. Catalyst Kinetics Chemistry.
From www.pinterest.ph
What Is a Catalyst? Understand Catalysis Chemical Reaction Catalyst Kinetics Chemistry Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. This file contains information regarding lecture 34. Learn about different types of catalysts in chemistry and their role in chemical reactions. Instead they provide a new mechanism for a reaction to. Catalysts allow a reaction to proceed via a pathway that has a. Catalyst Kinetics Chemistry.
From rumble.com
Catalysis, Chemistry Catalyst Kinetics Chemistry Instead they provide a new mechanism for a reaction to. Catalysts allow a reaction to. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts can lower the activation energy required for reactions, allowing them to proceed more quickly and efficiently. Learn about different types of catalysts in chemistry and their role. Catalyst Kinetics Chemistry.