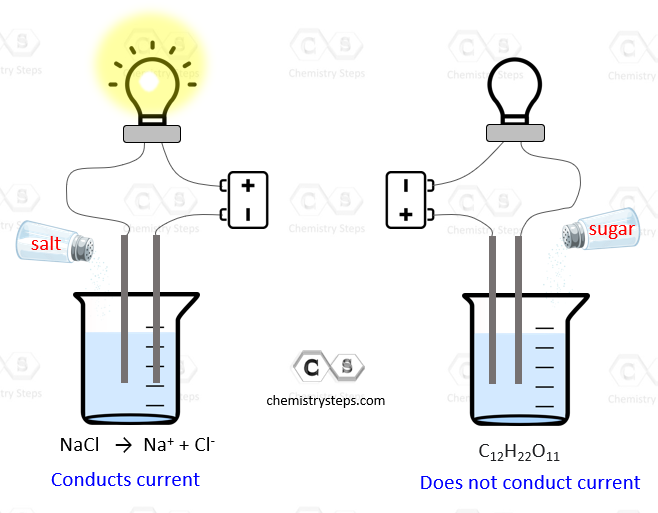
Can Nh4No3 Conduct Electricity In Water . Completely ionized or dissociate when dissolved in water (single arrow) a. We can also distinguish between these within the realm of. I feel that it should conduct electricity at the same. When dissolved, molecules of covalent compounds are separated from each other by. Thus, electrons flow like a “sea of electrons” through metals. It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. So how does a general good neutral electrolyte help water conduct electricity? Most covalent compounds are nonelectrolytes. Strong electrolytes conduct current very efficiently. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material.
from general.chemistrysteps.com
So how does a general good neutral electrolyte help water conduct electricity? Thus, electrons flow like a “sea of electrons” through metals. When dissolved, molecules of covalent compounds are separated from each other by. I feel that it should conduct electricity at the same. Most covalent compounds are nonelectrolytes. Completely ionized or dissociate when dissolved in water (single arrow) a. It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Strong electrolytes conduct current very efficiently.
General Properties of Solutions Chemistry Steps
Can Nh4No3 Conduct Electricity In Water Completely ionized or dissociate when dissolved in water (single arrow) a. Most covalent compounds are nonelectrolytes. Strong electrolytes conduct current very efficiently. So how does a general good neutral electrolyte help water conduct electricity? I feel that it should conduct electricity at the same. When dissolved, molecules of covalent compounds are separated from each other by. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. We can also distinguish between these within the realm of. It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. Thus, electrons flow like a “sea of electrons” through metals. Completely ionized or dissociate when dissolved in water (single arrow) a.
From www.youtube.com
Electrical conductivity with salt water YouTube Can Nh4No3 Conduct Electricity In Water Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. Thus, electrons flow like a “sea of electrons” through metals. We can also distinguish between these within the realm of. Completely ionized. Can Nh4No3 Conduct Electricity In Water.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps Can Nh4No3 Conduct Electricity In Water We can also distinguish between these within the realm of. I feel that it should conduct electricity at the same. When dissolved, molecules of covalent compounds are separated from each other by. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Strong electrolytes conduct current very efficiently. Completely ionized or dissociate. Can Nh4No3 Conduct Electricity In Water.
From www.youtube.com
Is NH4NO3 Acidic, Basic, or Neutral (Dissolved in Water)? YouTube Can Nh4No3 Conduct Electricity In Water Strong electrolytes conduct current very efficiently. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Most covalent compounds are nonelectrolytes. So how does a general good neutral electrolyte help water conduct electricity? When dissolved, molecules of covalent compounds are separated from each other by. I feel that it should conduct electricity. Can Nh4No3 Conduct Electricity In Water.
From www.youtube.com
Conductivity Of Water Distilled vs Brine Easy Science Experiments Can Nh4No3 Conduct Electricity In Water So how does a general good neutral electrolyte help water conduct electricity? Thus, electrons flow like a “sea of electrons” through metals. Strong electrolytes conduct current very efficiently. When dissolved, molecules of covalent compounds are separated from each other by. I feel that it should conduct electricity at the same. Metals are good conductors of electricity because they allow electrons. Can Nh4No3 Conduct Electricity In Water.
From www.slideserve.com
PPT Plan for Wed, 1 Oct 08 PowerPoint Presentation ID4479101 Can Nh4No3 Conduct Electricity In Water Completely ionized or dissociate when dissolved in water (single arrow) a. When dissolved, molecules of covalent compounds are separated from each other by. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Most covalent. Can Nh4No3 Conduct Electricity In Water.
From www.chegg.com
Solved When NH4NO3 is dissolved in water according to the Can Nh4No3 Conduct Electricity In Water Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. We can also distinguish between these within the realm of. So how does a general good neutral electrolyte help water conduct electricity? When dissolved, molecules. Can Nh4No3 Conduct Electricity In Water.
From ppt-online.org
Electrolytes. Reactions in Aqueous Solutions презентация онлайн Can Nh4No3 Conduct Electricity In Water Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Thus, electrons flow like a “sea of electrons” through metals. I feel that it should conduct electricity at the same. So how does a general. Can Nh4No3 Conduct Electricity In Water.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at Can Nh4No3 Conduct Electricity In Water Most covalent compounds are nonelectrolytes. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. When dissolved, molecules of covalent compounds are separated from each other by. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. Completely ionized or dissociate when dissolved in water. Can Nh4No3 Conduct Electricity In Water.
From www.youtube.com
How to Write the Net Ionic Equation for NH4NO3 + KOH = KNO3 + NH3 + H2O Can Nh4No3 Conduct Electricity In Water Thus, electrons flow like a “sea of electrons” through metals. Completely ionized or dissociate when dissolved in water (single arrow) a. When dissolved, molecules of covalent compounds are separated from each other by. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. So how does a general good neutral electrolyte help. Can Nh4No3 Conduct Electricity In Water.
From www.slideserve.com
PPT 22 Properties of water PowerPoint Presentation ID379006 Can Nh4No3 Conduct Electricity In Water We can also distinguish between these within the realm of. So how does a general good neutral electrolyte help water conduct electricity? When dissolved, molecules of covalent compounds are separated from each other by. I feel that it should conduct electricity at the same. Completely ionized or dissociate when dissolved in water (single arrow) a. Substances that dissolve in water. Can Nh4No3 Conduct Electricity In Water.
From www.researchgate.net
Prediction of surface tension of NH4NO3KNO3water ternary system using Can Nh4No3 Conduct Electricity In Water When dissolved, molecules of covalent compounds are separated from each other by. Thus, electrons flow like a “sea of electrons” through metals. Most covalent compounds are nonelectrolytes. Strong electrolytes conduct current very efficiently. I feel that it should conduct electricity at the same. We can also distinguish between these within the realm of. So how does a general good neutral. Can Nh4No3 Conduct Electricity In Water.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Can Nh4No3 Conduct Electricity In Water Strong electrolytes conduct current very efficiently. Completely ionized or dissociate when dissolved in water (single arrow) a. Most covalent compounds are nonelectrolytes. So how does a general good neutral electrolyte help water conduct electricity? When dissolved, molecules of covalent compounds are separated from each other by. I feel that it should conduct electricity at the same. We can also distinguish. Can Nh4No3 Conduct Electricity In Water.
From www.slideserve.com
PPT Aqueous Reactions and Solution Stoichiometry PowerPoint Can Nh4No3 Conduct Electricity In Water We can also distinguish between these within the realm of. When dissolved, molecules of covalent compounds are separated from each other by. Strong electrolytes conduct current very efficiently. Completely ionized or dissociate when dissolved in water (single arrow) a. Most covalent compounds are nonelectrolytes. Metals are good conductors of electricity because they allow electrons to flow through the entire piece. Can Nh4No3 Conduct Electricity In Water.
From sensorex.com
Why Electrical Conductivity of Water is Important for Industrial Can Nh4No3 Conduct Electricity In Water Completely ionized or dissociate when dissolved in water (single arrow) a. Strong electrolytes conduct current very efficiently. I feel that it should conduct electricity at the same. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Thus, electrons flow like a “sea of electrons” through metals. So how does a general. Can Nh4No3 Conduct Electricity In Water.
From www.teachoo.com
Do liquids conduct electricity? Class 8 Science Notes Teachoo Can Nh4No3 Conduct Electricity In Water Completely ionized or dissociate when dissolved in water (single arrow) a. It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. We can also distinguish between these within the realm of. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. Metals. Can Nh4No3 Conduct Electricity In Water.
From www.vrogue.co
Chemical Bonding Electrical Conductivity Of Ionic Com vrogue.co Can Nh4No3 Conduct Electricity In Water Thus, electrons flow like a “sea of electrons” through metals. Completely ionized or dissociate when dissolved in water (single arrow) a. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Strong electrolytes conduct current very efficiently. I feel that it should conduct electricity at the same. Substances that dissolve in water. Can Nh4No3 Conduct Electricity In Water.
From mungfali.com
Conductividad Electrica Del Agua Can Nh4No3 Conduct Electricity In Water Most covalent compounds are nonelectrolytes. We can also distinguish between these within the realm of. Strong electrolytes conduct current very efficiently. Thus, electrons flow like a “sea of electrons” through metals. It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. When dissolved, molecules of covalent compounds are separated. Can Nh4No3 Conduct Electricity In Water.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Can Nh4No3 Conduct Electricity In Water We can also distinguish between these within the realm of. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. I feel that it should conduct electricity at the same. When dissolved, molecules of covalent compounds are separated from each other by. Most covalent compounds are nonelectrolytes. Metals are good conductors of electricity. Can Nh4No3 Conduct Electricity In Water.
From www.youtube.com
Acids solution in water conducte elecricity YouTube Can Nh4No3 Conduct Electricity In Water When dissolved, molecules of covalent compounds are separated from each other by. So how does a general good neutral electrolyte help water conduct electricity? Most covalent compounds are nonelectrolytes. It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. Strong electrolytes conduct current very efficiently. Completely ionized or dissociate. Can Nh4No3 Conduct Electricity In Water.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Can Nh4No3 Conduct Electricity In Water Completely ionized or dissociate when dissolved in water (single arrow) a. Strong electrolytes conduct current very efficiently. Most covalent compounds are nonelectrolytes. So how does a general good neutral electrolyte help water conduct electricity? We can also distinguish between these within the realm of. I feel that it should conduct electricity at the same. Metals are good conductors of electricity. Can Nh4No3 Conduct Electricity In Water.
From slideplayer.com
The Gas Laws. ppt download Can Nh4No3 Conduct Electricity In Water So how does a general good neutral electrolyte help water conduct electricity? I feel that it should conduct electricity at the same. Most covalent compounds are nonelectrolytes. We can also distinguish between these within the realm of. Completely ionized or dissociate when dissolved in water (single arrow) a. Substances that dissolve in water can be categorized according to whether the. Can Nh4No3 Conduct Electricity In Water.
From www.slideserve.com
PPT Water, Electrolytes, and Solutions PowerPoint Presentation, free Can Nh4No3 Conduct Electricity In Water It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. Thus, electrons flow like a “sea of electrons” through metals. We can also distinguish between these within the realm of. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. Metals are. Can Nh4No3 Conduct Electricity In Water.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at Can Nh4No3 Conduct Electricity In Water Thus, electrons flow like a “sea of electrons” through metals. So how does a general good neutral electrolyte help water conduct electricity? Strong electrolytes conduct current very efficiently. When dissolved, molecules of covalent compounds are separated from each other by. I feel that it should conduct electricity at the same. It is known that while some liquids will conduct electricity. Can Nh4No3 Conduct Electricity In Water.
From www.youtube.com
Type of Reaction for HNO3 + NH3 = NH4NO3 YouTube Can Nh4No3 Conduct Electricity In Water Completely ionized or dissociate when dissolved in water (single arrow) a. It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. When dissolved, molecules of covalent compounds are separated from each. Can Nh4No3 Conduct Electricity In Water.
From www.youtube.com
Is NH4NO3 Soluble or Insoluble in Water? YouTube Can Nh4No3 Conduct Electricity In Water Most covalent compounds are nonelectrolytes. Strong electrolytes conduct current very efficiently. We can also distinguish between these within the realm of. I feel that it should conduct electricity at the same. So how does a general good neutral electrolyte help water conduct electricity? Metals are good conductors of electricity because they allow electrons to flow through the entire piece of. Can Nh4No3 Conduct Electricity In Water.
From www.rookieparenting.com
Does Water Conduct Electricity? Simple Experiment Can Nh4No3 Conduct Electricity In Water Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. I feel that it should conduct electricity at the same. So how does a general good neutral electrolyte help water conduct electricity? Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Most covalent compounds. Can Nh4No3 Conduct Electricity In Water.
From tipseri.com
What makes water a conductor of electricity? Tipseri Can Nh4No3 Conduct Electricity In Water So how does a general good neutral electrolyte help water conduct electricity? Most covalent compounds are nonelectrolytes. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. When dissolved, molecules of covalent compounds are separated from each other by. We can also distinguish between these within the realm of. Thus, electrons flow like. Can Nh4No3 Conduct Electricity In Water.
From www.researchgate.net
Correlation between N as NH4NO3 in water solution and electric Can Nh4No3 Conduct Electricity In Water We can also distinguish between these within the realm of. When dissolved, molecules of covalent compounds are separated from each other by. Strong electrolytes conduct current very efficiently. I feel that it should conduct electricity at the same. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. It is known that. Can Nh4No3 Conduct Electricity In Water.
From www.electricity-magnetism.org
¿Cómo conducen electricidad los electrolitos? Can Nh4No3 Conduct Electricity In Water Most covalent compounds are nonelectrolytes. It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. When dissolved, molecules of covalent compounds are separated from each other by. We can also distinguish between. Can Nh4No3 Conduct Electricity In Water.
From howtofunda.com
electrical conductivity experiment using saltwater Free Science Can Nh4No3 Conduct Electricity In Water I feel that it should conduct electricity at the same. When dissolved, molecules of covalent compounds are separated from each other by. Strong electrolytes conduct current very efficiently. Thus, electrons flow like a “sea of electrons” through metals. Completely ionized or dissociate when dissolved in water (single arrow) a. Substances that dissolve in water can be categorized according to whether. Can Nh4No3 Conduct Electricity In Water.
From www.slideserve.com
PPT Which of the following is evidence that a chemical reaction has Can Nh4No3 Conduct Electricity In Water Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. So how does a general good neutral electrolyte help water conduct electricity? Most covalent compounds are nonelectrolytes. When dissolved, molecules of covalent compounds are separated from each other by. I feel that it should conduct electricity at the same. Substances that dissolve. Can Nh4No3 Conduct Electricity In Water.
From www.youtube.com
ELECTRICAL CONDUCTIVITY OF LIQUIDS YouTube Can Nh4No3 Conduct Electricity In Water Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. Strong electrolytes conduct current very efficiently. It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. When dissolved, molecules of covalent compounds are separated from each other by. We can also distinguish. Can Nh4No3 Conduct Electricity In Water.
From lessonlibcocainises.z22.web.core.windows.net
Conducting Electricity With Salt Water Can Nh4No3 Conduct Electricity In Water It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. When dissolved, molecules of covalent compounds are separated from each other by. So how does a general good neutral electrolyte help water conduct electricity? Completely ionized or dissociate when dissolved in water (single arrow) a. Strong electrolytes conduct current. Can Nh4No3 Conduct Electricity In Water.
From learningmediablotted.z21.web.core.windows.net
Water Is Conductor Of Electricity Can Nh4No3 Conduct Electricity In Water Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. I feel that it should conduct electricity at the same. Completely ionized or dissociate when dissolved in water (single arrow) a. Thus, electrons flow like. Can Nh4No3 Conduct Electricity In Water.
From msestudent.com
Why Do Metals Conduct Electricity? Materials Science & Engineering Can Nh4No3 Conduct Electricity In Water Thus, electrons flow like a “sea of electrons” through metals. Strong electrolytes conduct current very efficiently. So how does a general good neutral electrolyte help water conduct electricity? Completely ionized or dissociate when dissolved in water (single arrow) a. Metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. We can also. Can Nh4No3 Conduct Electricity In Water.