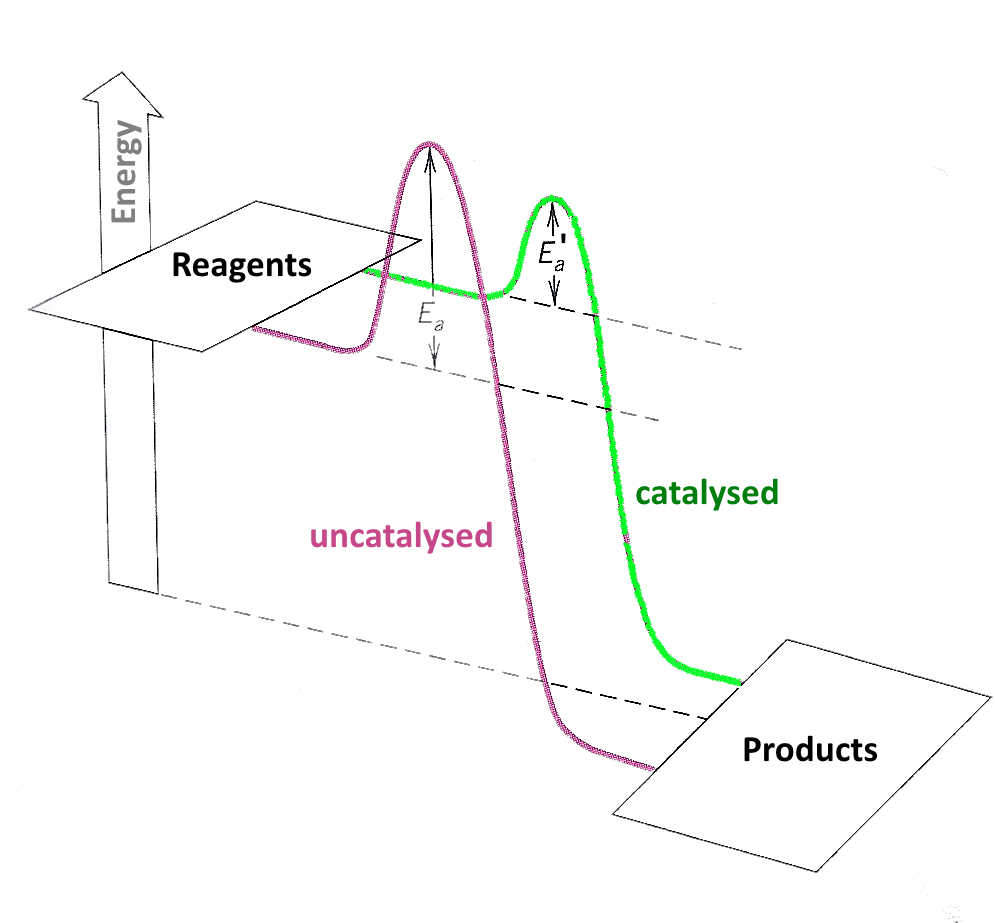
Catalysts Decrease The Energy . A catalyst reduces the activation energy needed for a reaction. To increase the rate of a reaction you need to increase the number of successful collisions. A catalyst speeds up the rate of a reaction by lowering the activation energy; A catalyst speeds up the rate of a reaction by lowering the activation energy; List examples of catalysis in natural. In addition, the catalyst is regenerated in the process. A catalyst provides an alternative route for the reaction with a lower activation energy. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. It does not lower the activation energy of the reaction. With a catalyst present, more collisions between reactants result in the. One possible way of doing this is to provide an alternative way for. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Several reactions that are thermodynamically. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. In addition, the catalyst is regenerated in the process.
from www.chim.lu
Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. In addition, the catalyst is regenerated in the process. With a catalyst present, more collisions between reactants result in the. A catalyst provides an alternative route for the reaction with a lower activation energy. Several reactions that are thermodynamically. List examples of catalysis in natural. It does not lower the activation energy of the reaction. To increase the rate of a reaction you need to increase the number of successful collisions. There is a subtle difference between the two statements that is easily illustrated with a simple analogy.
Activation energy and catalysis
Catalysts Decrease The Energy Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. To increase the rate of a reaction you need to increase the number of successful collisions. A catalyst reduces the activation energy needed for a reaction. A catalyst provides an alternative route for the reaction with a lower activation energy. In addition, the catalyst is regenerated in the process. In addition, the catalyst is regenerated in the process. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. One possible way of doing this is to provide an alternative way for. It does not lower the activation energy of the reaction. A catalyst speeds up the rate of a reaction by lowering the activation energy; Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. A catalyst speeds up the rate of a reaction by lowering the activation energy; List examples of catalysis in natural. Several reactions that are thermodynamically. With a catalyst present, more collisions between reactants result in the.
From www.sliderbase.com
Catalysis Presentation Chemistry Catalysts Decrease The Energy A catalyst speeds up the rate of a reaction by lowering the activation energy; List examples of catalysis in natural. A catalyst speeds up the rate of a reaction by lowering the activation energy; It does not lower the activation energy of the reaction. There is a subtle difference between the two statements that is easily illustrated with a simple. Catalysts Decrease The Energy.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID1835084 Catalysts Decrease The Energy Several reactions that are thermodynamically. It does not lower the activation energy of the reaction. A catalyst reduces the activation energy needed for a reaction. One possible way of doing this is to provide an alternative way for. A catalyst speeds up the rate of a reaction by lowering the activation energy; A catalyst provides an alternative route for the. Catalysts Decrease The Energy.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalysts Decrease The Energy One possible way of doing this is to provide an alternative way for. To increase the rate of a reaction you need to increase the number of successful collisions. It does not lower the activation energy of the reaction. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. In addition, the catalyst. Catalysts Decrease The Energy.
From slideplayer.com
Enzymes. ppt download Catalysts Decrease The Energy It does not lower the activation energy of the reaction. To increase the rate of a reaction you need to increase the number of successful collisions. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst provides an alternative route for the reaction with a lower activation energy. In addition, the catalyst is. Catalysts Decrease The Energy.
From www.workmanagementsolutions.com.au
An Analogy The Activation Energy and Catalysis in Business Operations Catalysts Decrease The Energy A catalyst reduces the activation energy needed for a reaction. List examples of catalysis in natural. In addition, the catalyst is regenerated in the process. It does not lower the activation energy of the reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst provides an alternative route for the reaction with. Catalysts Decrease The Energy.
From byjus.com
How does a catalyst increase the rate of a reaction? Catalysts Decrease The Energy One possible way of doing this is to provide an alternative way for. In addition, the catalyst is regenerated in the process. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. With a catalyst present, more collisions between reactants result in the. A catalyst speeds up the rate of a reaction by. Catalysts Decrease The Energy.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Catalysts Decrease The Energy A catalyst speeds up the rate of a reaction by lowering the activation energy; A catalyst provides an alternative route for the reaction with a lower activation energy. Several reactions that are thermodynamically. In addition, the catalyst is regenerated in the process. List examples of catalysis in natural. There is a subtle difference between the two statements that is easily. Catalysts Decrease The Energy.
From www.researchgate.net
Schematic illustration showing the strategies to improve the catalytic Catalysts Decrease The Energy A catalyst reduces the activation energy needed for a reaction. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. In addition, the catalyst is regenerated in the process. Explain the function of a catalyst in terms. Catalysts Decrease The Energy.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalysts Decrease The Energy It does not lower the activation energy of the reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst reduces the activation energy needed for a reaction. A catalyst speeds up the rate of a reaction by lowering the activation energy; To increase the rate of a reaction you need to increase. Catalysts Decrease The Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Decrease The Energy A catalyst provides an alternative route for the reaction with a lower activation energy. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. A catalyst reduces the activation energy needed for a reaction. To increase the rate of a reaction you need to increase the number of successful collisions. Several reactions that. Catalysts Decrease The Energy.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalysts Decrease The Energy Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. To increase the rate of a reaction you need to increase the number of successful collisions. In addition, the catalyst is regenerated in the process. List examples of. Catalysts Decrease The Energy.
From www.chim.lu
Activation energy and catalysis Catalysts Decrease The Energy In addition, the catalyst is regenerated in the process. One possible way of doing this is to provide an alternative way for. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. To increase the rate of a reaction you need to increase the number of successful collisions. A catalyst provides an alternative. Catalysts Decrease The Energy.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalysts Decrease The Energy A catalyst reduces the activation energy needed for a reaction. Several reactions that are thermodynamically. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst speeds up the rate of a reaction by lowering the activation energy; It does not lower the activation energy of the reaction. A catalyst provides an alternative route. Catalysts Decrease The Energy.
From byjus.com
Reaction Coordinate Diagram An Overview of Reaction Coordinate Catalysts Decrease The Energy In addition, the catalyst is regenerated in the process. Several reactions that are thermodynamically. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. With a catalyst present, more collisions between reactants result in the. A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst speeds up. Catalysts Decrease The Energy.
From 2012books.lardbucket.org
Catalysis Catalysts Decrease The Energy To increase the rate of a reaction you need to increase the number of successful collisions. List examples of catalysis in natural. In addition, the catalyst is regenerated in the process. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. In addition, the catalyst is regenerated in the process. Explain. Catalysts Decrease The Energy.
From www.nagwa.com
Question Video Determining How a Catalyst Affects the Energy Profile Catalysts Decrease The Energy List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. In addition, the catalyst is regenerated in the process. To increase the rate of a reaction you need to increase the number of successful collisions. Catalysts function by allowing the reaction to take place through an alternative mechanism that. Catalysts Decrease The Energy.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Catalysts Decrease The Energy A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst speeds up the rate of a reaction by lowering the activation energy; A catalyst speeds up the rate of a reaction by lowering the activation energy; A catalyst reduces the activation energy needed for a reaction. With a catalyst present, more collisions between reactants. Catalysts Decrease The Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Catalysts Decrease The Energy A catalyst speeds up the rate of a reaction by lowering the activation energy; Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst provides an alternative route for the reaction with a lower activation energy. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller. Catalysts Decrease The Energy.
From www.meritnation.com
When a catalyst is added to a system at equilibrium, a decrease occurs Catalysts Decrease The Energy In addition, the catalyst is regenerated in the process. Several reactions that are thermodynamically. A catalyst reduces the activation energy needed for a reaction. To increase the rate of a reaction you need to increase the number of successful collisions. In addition, the catalyst is regenerated in the process. Catalysts function by allowing the reaction to take place through an. Catalysts Decrease The Energy.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalysts Decrease The Energy A catalyst reduces the activation energy needed for a reaction. A catalyst speeds up the rate of a reaction by lowering the activation energy; One possible way of doing this is to provide an alternative way for. A catalyst provides an alternative route for the reaction with a lower activation energy. List examples of catalysis in natural. With a catalyst. Catalysts Decrease The Energy.
From slideplayer.com
& Equilibrium ppt download Catalysts Decrease The Energy To increase the rate of a reaction you need to increase the number of successful collisions. It does not lower the activation energy of the reaction. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller. Catalysts Decrease The Energy.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalysts Decrease The Energy It does not lower the activation energy of the reaction. A catalyst speeds up the rate of a reaction by lowering the activation energy; One possible way of doing this is to provide an alternative way for. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. A catalyst speeds up. Catalysts Decrease The Energy.
From slideplayer.com
A catalyst lowers activation energy. ppt download Catalysts Decrease The Energy A catalyst reduces the activation energy needed for a reaction. List examples of catalysis in natural. To increase the rate of a reaction you need to increase the number of successful collisions. A catalyst speeds up the rate of a reaction by lowering the activation energy; Several reactions that are thermodynamically. With a catalyst present, more collisions between reactants result. Catalysts Decrease The Energy.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysts Decrease The Energy One possible way of doing this is to provide an alternative way for. List examples of catalysis in natural. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. It does not lower the activation energy of the reaction. A catalyst reduces the activation energy needed for a reaction. In addition, the catalyst. Catalysts Decrease The Energy.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint Catalysts Decrease The Energy With a catalyst present, more collisions between reactants result in the. In addition, the catalyst is regenerated in the process. A catalyst speeds up the rate of a reaction by lowering the activation energy; In addition, the catalyst is regenerated in the process. A catalyst speeds up the rate of a reaction by lowering the activation energy; A catalyst reduces. Catalysts Decrease The Energy.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalysts Decrease The Energy Several reactions that are thermodynamically. To increase the rate of a reaction you need to increase the number of successful collisions. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. List examples of catalysis in natural. A catalyst reduces the activation energy needed for a reaction. Explain the function of. Catalysts Decrease The Energy.
From slideplayer.com
Chapter 24 Chemical Reactions and Enzymes. ppt download Catalysts Decrease The Energy One possible way of doing this is to provide an alternative way for. In addition, the catalyst is regenerated in the process. A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst speeds up the rate of a reaction by lowering the activation energy; It does not lower the activation energy of the reaction.. Catalysts Decrease The Energy.
From www.catalystseurope.org
What are catalysts? Catalysts Decrease The Energy To increase the rate of a reaction you need to increase the number of successful collisions. In addition, the catalyst is regenerated in the process. List examples of catalysis in natural. It does not lower the activation energy of the reaction. There is a subtle difference between the two statements that is easily illustrated with a simple analogy. Explain the. Catalysts Decrease The Energy.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Catalysts Decrease The Energy A catalyst speeds up the rate of a reaction by lowering the activation energy; A catalyst speeds up the rate of a reaction by lowering the activation energy; Several reactions that are thermodynamically. A catalyst reduces the activation energy needed for a reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. In addition,. Catalysts Decrease The Energy.
From slideplayer.com
Lecture 1405 Reaction Mechanism and Catalysis ppt download Catalysts Decrease The Energy A catalyst speeds up the rate of a reaction by lowering the activation energy; It does not lower the activation energy of the reaction. One possible way of doing this is to provide an alternative way for. With a catalyst present, more collisions between reactants result in the. Explain the function of a catalyst in terms of reaction mechanisms and. Catalysts Decrease The Energy.
From www.slideserve.com
PPT The Collision Theory and Activation Energy PowerPoint Catalysts Decrease The Energy Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. It does not lower the activation energy of the reaction. List examples of catalysis in natural. Several reactions that are thermodynamically. In addition, the catalyst is regenerated in the process. A catalyst speeds up the rate of a reaction by lowering the activation energy; There. Catalysts Decrease The Energy.
From large.stanford.edu
Catalysts in 21st Century Energy Catalysts Decrease The Energy A catalyst speeds up the rate of a reaction by lowering the activation energy; A catalyst provides an alternative route for the reaction with a lower activation energy. It does not lower the activation energy of the reaction. One possible way of doing this is to provide an alternative way for. Several reactions that are thermodynamically. A catalyst reduces the. Catalysts Decrease The Energy.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts Decrease The Energy In addition, the catalyst is regenerated in the process. It does not lower the activation energy of the reaction. List examples of catalysis in natural. With a catalyst present, more collisions between reactants result in the. To increase the rate of a reaction you need to increase the number of successful collisions. In addition, the catalyst is regenerated in the. Catalysts Decrease The Energy.
From slideplayer.com
CHAPTER 5 The Working Cell Energy and Enzymes ppt download Catalysts Decrease The Energy A catalyst reduces the activation energy needed for a reaction. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst speeds up the rate of a reaction by lowering the activation energy; Several reactions that. Catalysts Decrease The Energy.
From slideplayer.com
Day 6 Chemical Equilibrium ppt download Catalysts Decrease The Energy One possible way of doing this is to provide an alternative way for. A catalyst speeds up the rate of a reaction by lowering the activation energy; There is a subtle difference between the two statements that is easily illustrated with a simple analogy. A catalyst reduces the activation energy needed for a reaction. List examples of catalysis in natural.. Catalysts Decrease The Energy.