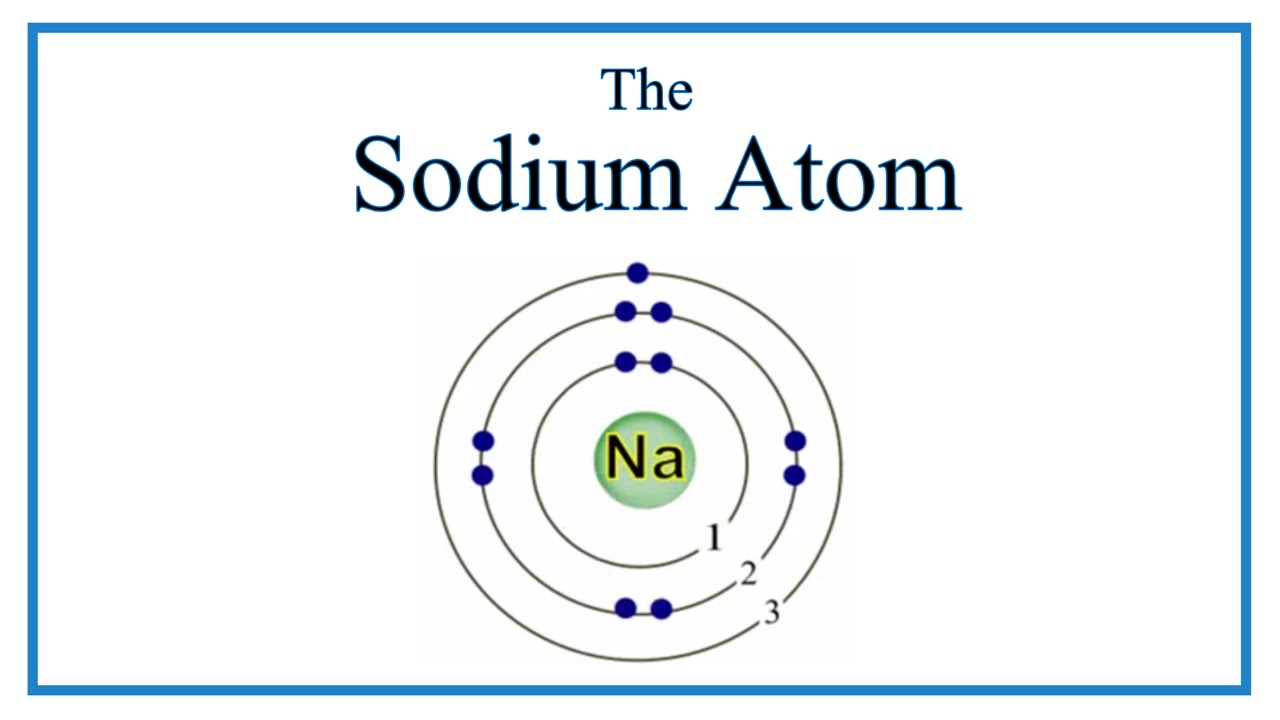
Does Sodium Have A Negative Charge . 93 rows ionic charge: As you have learned, ions are atoms or molecules bearing an electrical charge. This electric charge generated on the ion is. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. For example, sodium is an element with 11 protons within the nucleus and 11 electrons. The names for positive and negative ions are pronounced cat. Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons. You can use this table to predict whether an atom can bond with another atom. 93 rows this table shows the most common charges for atoms of the chemical elements. A cation (a positive ion) forms when a neutral atom loses one or. Another example of an element is carbon, which has six protons within the nucleus and. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Thus, if you commit the information in table.
from www.youtube.com
When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. 93 rows this table shows the most common charges for atoms of the chemical elements. As you have learned, ions are atoms or molecules bearing an electrical charge. This electric charge generated on the ion is. A cation (a positive ion) forms when a neutral atom loses one or. Thus, if you commit the information in table. The names for positive and negative ions are pronounced cat. Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons. You can use this table to predict whether an atom can bond with another atom. For example, sodium is an element with 11 protons within the nucleus and 11 electrons.
Atomic Structure of the Sodium Atom (Na) YouTube
Does Sodium Have A Negative Charge For example, sodium is an element with 11 protons within the nucleus and 11 electrons. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Thus, if you commit the information in table. This electric charge generated on the ion is. 93 rows this table shows the most common charges for atoms of the chemical elements. Another example of an element is carbon, which has six protons within the nucleus and. A cation (a positive ion) forms when a neutral atom loses one or. You can use this table to predict whether an atom can bond with another atom. Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons. For example, sodium is an element with 11 protons within the nucleus and 11 electrons. As you have learned, ions are atoms or molecules bearing an electrical charge. The names for positive and negative ions are pronounced cat.
From periodictable.me
Sodium Valence Electrons Sodium Valency (Na) with Dot Diagram Does Sodium Have A Negative Charge You can use this table to predict whether an atom can bond with another atom. Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons. For example, sodium is an element with 11 protons within the nucleus and 11 electrons. As you have learned, ions are atoms or molecules. Does Sodium Have A Negative Charge.
From animalia-life.club
Sodium Shell Model Does Sodium Have A Negative Charge This electric charge generated on the ion is. A cation (a positive ion) forms when a neutral atom loses one or. 93 rows ionic charge: When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Another example of an element is carbon, which has six protons within the nucleus and.. Does Sodium Have A Negative Charge.
From www.pikpng.com
Download Png Royalty Free Drawing Atoms Sodium Covalent Bonding Of Sodium Chloride Clipart Png Does Sodium Have A Negative Charge When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. A cation (a positive ion) forms when a neutral atom loses one or. Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons. As you have learned, ions are. Does Sodium Have A Negative Charge.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Does Sodium Have A Negative Charge 93 rows this table shows the most common charges for atoms of the chemical elements. This electric charge generated on the ion is. You can use this table to predict whether an atom can bond with another atom. Thus, if you commit the information in table. Another example of an element is carbon, which has six protons within the nucleus. Does Sodium Have A Negative Charge.
From courses.lumenlearning.com
3.2 Ions The Basics of General, Organic, and Biological Chemistry Does Sodium Have A Negative Charge 93 rows ionic charge: For example, sodium is an element with 11 protons within the nucleus and 11 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges for atoms of the chemical elements. Sodium atoms have 11 protons (because. Does Sodium Have A Negative Charge.
From jasmine-has-gillespie.blogspot.com
Sodium Forms an Ion With a Charge of JasminehasGillespie Does Sodium Have A Negative Charge 93 rows this table shows the most common charges for atoms of the chemical elements. Another example of an element is carbon, which has six protons within the nucleus and. For example, sodium is an element with 11 protons within the nucleus and 11 electrons. Thus, if you commit the information in table. This electric charge generated on the ion. Does Sodium Have A Negative Charge.
From ar.inspiredpencil.com
Sodium Atom With 11 Protons And 11 Neutrons Does Sodium Have A Negative Charge For example, sodium is an element with 11 protons within the nucleus and 11 electrons. A cation (a positive ion) forms when a neutral atom loses one or. 93 rows this table shows the most common charges for atoms of the chemical elements. The names for positive and negative ions are pronounced cat. Sodium atoms have 11 protons (because the. Does Sodium Have A Negative Charge.
From www.numerade.com
SOLVED Balancing chemical formulas Balance the charges on the ions to form ionic compounds Does Sodium Have A Negative Charge 93 rows ionic charge: As you have learned, ions are atoms or molecules bearing an electrical charge. You can use this table to predict whether an atom can bond with another atom. Thus, if you commit the information in table. Another example of an element is carbon, which has six protons within the nucleus and. 93 rows this table shows. Does Sodium Have A Negative Charge.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent Does Sodium Have A Negative Charge The names for positive and negative ions are pronounced cat. Thus, if you commit the information in table. 93 rows ionic charge: This electric charge generated on the ion is. Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons. You can use this table to predict whether an. Does Sodium Have A Negative Charge.
From www.vrogue.co
Sodium Ion Na Chemspider vrogue.co Does Sodium Have A Negative Charge 93 rows ionic charge: 93 rows this table shows the most common charges for atoms of the chemical elements. For example, sodium is an element with 11 protons within the nucleus and 11 electrons. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Thus, if you commit the information. Does Sodium Have A Negative Charge.
From ar.inspiredpencil.com
Sodium Potassium Pump Excess Charge Does Sodium Have A Negative Charge When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. This electric charge generated on the ion is. 93 rows ionic charge: Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons. A cation (a positive ion) forms when. Does Sodium Have A Negative Charge.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 Does Sodium Have A Negative Charge 93 rows this table shows the most common charges for atoms of the chemical elements. As you have learned, ions are atoms or molecules bearing an electrical charge. Another example of an element is carbon, which has six protons within the nucleus and. For example, sodium is an element with 11 protons within the nucleus and 11 electrons. Sodium atoms. Does Sodium Have A Negative Charge.
From www.youtube.com
Atomic Structure of the Sodium Atom (Na) YouTube Does Sodium Have A Negative Charge 93 rows this table shows the most common charges for atoms of the chemical elements. Another example of an element is carbon, which has six protons within the nucleus and. Thus, if you commit the information in table. A cation (a positive ion) forms when a neutral atom loses one or. This electric charge generated on the ion is. As. Does Sodium Have A Negative Charge.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Does Sodium Have A Negative Charge For example, sodium is an element with 11 protons within the nucleus and 11 electrons. As you have learned, ions are atoms or molecules bearing an electrical charge. 93 rows ionic charge: Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons. When sodium atoms form ions, they always. Does Sodium Have A Negative Charge.
From mavink.com
Sodium Atom Protons Neutrons Electrons Does Sodium Have A Negative Charge For example, sodium is an element with 11 protons within the nucleus and 11 electrons. The names for positive and negative ions are pronounced cat. Another example of an element is carbon, which has six protons within the nucleus and. As you have learned, ions are atoms or molecules bearing an electrical charge. When the atom loses or gains one. Does Sodium Have A Negative Charge.
From sciencing.com
How to Calculate the Charge of an Ion Sciencing Does Sodium Have A Negative Charge This electric charge generated on the ion is. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). You can use this table to predict whether an atom can bond. Does Sodium Have A Negative Charge.
From www.youtube.com
How to Find the Ionic Charge for Sodium (Na) YouTube Does Sodium Have A Negative Charge The names for positive and negative ions are pronounced cat. This electric charge generated on the ion is. For example, sodium is an element with 11 protons within the nucleus and 11 electrons. You can use this table to predict whether an atom can bond with another atom. Thus, if you commit the information in table. 93 rows this table. Does Sodium Have A Negative Charge.
From openbooks.lib.msu.edu
Membrane Potential Foundations of Neuroscience Does Sodium Have A Negative Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). For example, sodium is an element with 11 protons within the nucleus and 11 electrons. Another example of an element is carbon, which has six protons within the nucleus and. This electric charge generated on the ion is. 93 rows. Does Sodium Have A Negative Charge.
From infraredforhealth.com
How Do You Explain Negative Charge? Infrared for Health Does Sodium Have A Negative Charge A cation (a positive ion) forms when a neutral atom loses one or. 93 rows ionic charge: As you have learned, ions are atoms or molecules bearing an electrical charge. You can use this table to predict whether an atom can bond with another atom. For example, sodium is an element with 11 protons within the nucleus and 11 electrons.. Does Sodium Have A Negative Charge.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Does Sodium Have A Negative Charge For example, sodium is an element with 11 protons within the nucleus and 11 electrons. 93 rows this table shows the most common charges for atoms of the chemical elements. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. A cation (a positive ion) forms when a neutral atom. Does Sodium Have A Negative Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Does Sodium Have A Negative Charge 93 rows this table shows the most common charges for atoms of the chemical elements. Thus, if you commit the information in table. For example, sodium is an element with 11 protons within the nucleus and 11 electrons. The names for positive and negative ions are pronounced cat. A cation (a positive ion) forms when a neutral atom loses one. Does Sodium Have A Negative Charge.
From www.nuclear-power.com
Sodium Atomic Number Atomic Mass Density of Sodium Does Sodium Have A Negative Charge When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. 93 rows ionic charge: 93 rows this table shows the most common charges for atoms of the chemical elements. The names for positive and negative ions are pronounced cat. A cation (a positive ion) forms when a neutral atom loses. Does Sodium Have A Negative Charge.
From valenceelectrons.com
How Many Valence Electrons Does Sodium(Na) Have? Does Sodium Have A Negative Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges for atoms of the chemical elements. This electric charge generated on the ion is. Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because. Does Sodium Have A Negative Charge.
From courses.lumenlearning.com
2.7 Molecular and Ionic Compounds Chemistry Does Sodium Have A Negative Charge You can use this table to predict whether an atom can bond with another atom. Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons. 93 rows ionic charge: For example, sodium is an element with 11 protons within the nucleus and 11 electrons. This electric charge generated on. Does Sodium Have A Negative Charge.
From www.researchgate.net
Interaction of a positive charge (sodium ion) and a negative charge... Download Scientific Diagram Does Sodium Have A Negative Charge 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). You can use this table to predict whether an atom can bond with another atom. A cation (a positive ion) forms when a neutral atom loses one or. 93 rows this table shows the most common. Does Sodium Have A Negative Charge.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Does Sodium Have A Negative Charge 93 rows this table shows the most common charges for atoms of the chemical elements. 93 rows ionic charge: You can use this table to predict whether an atom can bond with another atom. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. As you have learned, ions are. Does Sodium Have A Negative Charge.
From www.youtube.com
How many valence electrons does sodium have?How to find the valence electrons for sodium (Na Does Sodium Have A Negative Charge 93 rows this table shows the most common charges for atoms of the chemical elements. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Another example of an element. Does Sodium Have A Negative Charge.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Does Sodium Have A Negative Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). A cation (a positive ion) forms when a neutral atom loses one or. For example, sodium is an element with 11 protons within the nucleus and 11 electrons. You can use this table to predict whether an atom can bond. Does Sodium Have A Negative Charge.
From www.youtube.com
How to find Protons & Electrons for the Sodium ion (Na+) YouTube Does Sodium Have A Negative Charge As you have learned, ions are atoms or molecules bearing an electrical charge. Another example of an element is carbon, which has six protons within the nucleus and. For example, sodium is an element with 11 protons within the nucleus and 11 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an. Does Sodium Have A Negative Charge.
From courses.lumenlearning.com
Lewis Symbols and Structures Chemistry Does Sodium Have A Negative Charge Thus, if you commit the information in table. You can use this table to predict whether an atom can bond with another atom. This electric charge generated on the ion is. As you have learned, ions are atoms or molecules bearing an electrical charge. Another example of an element is carbon, which has six protons within the nucleus and. When. Does Sodium Have A Negative Charge.
From www.numerade.com
How does the sodiumpotassium pump contribute to the net negative charge of the interior of the Does Sodium Have A Negative Charge This electric charge generated on the ion is. Thus, if you commit the information in table. 93 rows ionic charge: A cation (a positive ion) forms when a neutral atom loses one or. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When sodium atoms form ions, they always. Does Sodium Have A Negative Charge.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Does Sodium Have A Negative Charge As you have learned, ions are atoms or molecules bearing an electrical charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Thus, if you commit the information in table. You can use this table to predict whether an atom can bond with another atom. 93 rows this table. Does Sodium Have A Negative Charge.
From www.nagwa.com
Question Video Identifying the Statement That Best Describes the Structure of Sodium Chloride Does Sodium Have A Negative Charge 93 rows ionic charge: A cation (a positive ion) forms when a neutral atom loses one or. Another example of an element is carbon, which has six protons within the nucleus and. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. For example, sodium is an element with 11. Does Sodium Have A Negative Charge.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind the Atoms Together Does Sodium Have A Negative Charge Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Thus, if you commit the information in table. A cation (a positive ion) forms when a neutral atom loses. Does Sodium Have A Negative Charge.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download ID7021047 Does Sodium Have A Negative Charge This electric charge generated on the ion is. Another example of an element is carbon, which has six protons within the nucleus and. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: When sodium atoms form ions, they always form a 1+ charge, never a. Does Sodium Have A Negative Charge.