Lead Nitrate Relative Formula Mass . [2] the molar mass of oxygen is 15.999 g/mol. Convert grams lead nitrate to moles. [1] the molar mass of nitrogen is 14.007 g/mol. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. Compute mass of each element. It will calculate the total mass along with the elemental composition and mass of each. [3] now, to calculate the molar mass of pb. (a r of mg =. This site explains how to. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass. Molar mass of pb(no3)2 = 331.2098 g/mol. Enter the molecular formula of the substance. The molar mass of lead is 207.2 g/mol. Track your food intake, exercise, sleep and meditation for.
from www.chegg.com
Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass. Track your food intake, exercise, sleep and meditation for. [2] the molar mass of oxygen is 15.999 g/mol. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. Enter the molecular formula of the substance. [1] the molar mass of nitrogen is 14.007 g/mol. [3] now, to calculate the molar mass of pb. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. (a r of mg =. Molar mass of pb(no3)2 = 331.2098 g/mol.
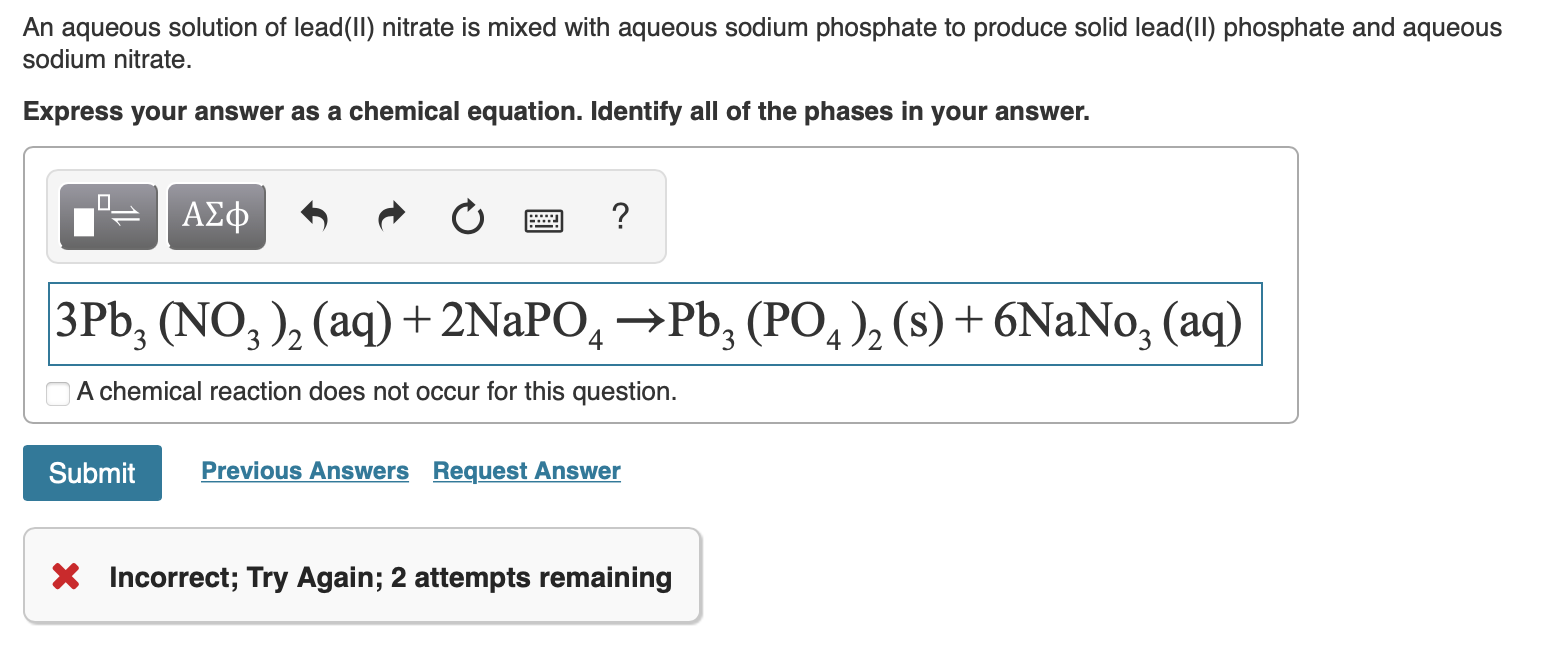
Solved An aqueous solution of lead(II) nitrate is mixed with
Lead Nitrate Relative Formula Mass [3] now, to calculate the molar mass of pb. Molar mass of pb(no3)2 = 331.2098 g/mol. (a r of mg =. It will calculate the total mass along with the elemental composition and mass of each. [2] the molar mass of oxygen is 15.999 g/mol. Compute mass of each element. Track your food intake, exercise, sleep and meditation for. [3] now, to calculate the molar mass of pb. This site explains how to. The molar mass of lead is 207.2 g/mol. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. [1] the molar mass of nitrogen is 14.007 g/mol. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. Enter the molecular formula of the substance. Convert grams lead nitrate to moles.
From slideplayer.com
Types of Chemical Reactions Ch ppt download Lead Nitrate Relative Formula Mass Molar mass of pb(no3)2 = 331.2098 g/mol. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. [1] the molar mass of nitrogen is 14.007 g/mol. (a r of mg =. [2] the molar mass of oxygen is 15.999 g/mol. Multiply the number of atoms by the atomic weight of each element found in steps 1 and. Lead Nitrate Relative Formula Mass.
From www.meritnation.com
write the formula for lead nitrate by criss cross method Science Lead Nitrate Relative Formula Mass It will calculate the total mass along with the elemental composition and mass of each. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. This site explains how to. Convert grams lead nitrate to. Lead Nitrate Relative Formula Mass.
From www.chegg.com
Solved An aqueous solution of lead(II) nitrate is mixed with Lead Nitrate Relative Formula Mass The molar mass of lead is 207.2 g/mol. [1] the molar mass of nitrogen is 14.007 g/mol. Enter the molecular formula of the substance. Molar mass of pb(no3)2 = 331.2098 g/mol. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. Convert grams lead nitrate to moles. It will calculate the total mass along with the elemental. Lead Nitrate Relative Formula Mass.
From brainly.in
a) what is the formula of lead nitrate? b) Name the products formed on Lead Nitrate Relative Formula Mass Compute mass of each element. (a r of mg =. [3] now, to calculate the molar mass of pb. It will calculate the total mass along with the elemental composition and mass of each. This site explains how to. Track your food intake, exercise, sleep and meditation for. Molar mass of pb(no3)2 = 331.2098 g/mol. Enter the molecular formula of. Lead Nitrate Relative Formula Mass.
From www.studocu.com
Data385361566852108 The general path for this calculation is Find Lead Nitrate Relative Formula Mass Convert grams lead nitrate to moles. (a r of mg =. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass. Compute mass of each element. Molar mass of pb(no3)2 = 331.2098 g/mol. The molar mass of lead is 207.2 g/mol. [1] the molar mass of nitrogen is. Lead Nitrate Relative Formula Mass.
From www.toppr.com
If the relative molecular mass of ammonium nitrate is 80, the Lead Nitrate Relative Formula Mass This site explains how to. The molar mass of lead is 207.2 g/mol. [1] the molar mass of nitrogen is 14.007 g/mol. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. Convert grams lead nitrate to moles. [3] now, to calculate the molar mass of pb. It will calculate the total mass along with the elemental. Lead Nitrate Relative Formula Mass.
From www.youtube.com
IGCSE Chemistry lesson 34 part c Thermal of nitrates Lead Nitrate Relative Formula Mass Track your food intake, exercise, sleep and meditation for. (a r of mg =. Enter the molecular formula of the substance. Convert grams lead nitrate to moles. [3] now, to calculate the molar mass of pb. It will calculate the total mass along with the elemental composition and mass of each. Compute mass of each element. Molar mass of lead. Lead Nitrate Relative Formula Mass.
From askfilo.com
What is the Chemical formula of? lead Nitrate ii) Potassium lodide ii) Le.. Lead Nitrate Relative Formula Mass [3] now, to calculate the molar mass of pb. (a r of mg =. The molar mass of lead is 207.2 g/mol. Track your food intake, exercise, sleep and meditation for. Molar mass of pb(no3)2 = 331.2098 g/mol. Convert grams lead nitrate to moles. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. This site explains. Lead Nitrate Relative Formula Mass.
From www.youtube.com
Write the balanced chemical equation for each of the following Lead Nitrate Relative Formula Mass Compute mass of each element. This site explains how to. It will calculate the total mass along with the elemental composition and mass of each. [1] the molar mass of nitrogen is 14.007 g/mol. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol.. Lead Nitrate Relative Formula Mass.
From mccnsulting.web.fc2.com
What happens when lead nitrate is heated? Lead Nitrate Relative Formula Mass Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. [2] the molar mass of oxygen is 15.999 g/mol. Molar mass of pb(no3)2 = 331.2098 g/mol. (a r of mg =. [1] the molar mass of nitrogen is 14.007 g/mol. Multiply the number of atoms by the atomic weight of each element found in steps 1 and. Lead Nitrate Relative Formula Mass.
From kolblabs.com
Heating of Lead nitrate in Chemical Reactions and Equations Class 10 Lead Nitrate Relative Formula Mass (a r of mg =. [2] the molar mass of oxygen is 15.999 g/mol. Enter the molecular formula of the substance. It will calculate the total mass along with the elemental composition and mass of each. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no. Lead Nitrate Relative Formula Mass.
From askfilo.com
Lead nitrate [Pb(NO3 )2 ] on reaction with sodium lodide [Nal] form ppt o.. Lead Nitrate Relative Formula Mass Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. [1] the molar mass of nitrogen is 14.007 g/mol. Track your food intake, exercise, sleep and meditation for. [3] now, to calculate the molar mass of pb. (a r of mg =. Compute mass of each element. [2] the molar mass of oxygen is 15.999 g/mol. Molar. Lead Nitrate Relative Formula Mass.
From sciencelab.com
Lead Nitrate, Crystal, Reagent, ACS Grade Lead Nitrate Relative Formula Mass [1] the molar mass of nitrogen is 14.007 g/mol. (a r of mg =. Molar mass of pb(no3)2 = 331.2098 g/mol. [2] the molar mass of oxygen is 15.999 g/mol. It will calculate the total mass along with the elemental composition and mass of each. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. This site. Lead Nitrate Relative Formula Mass.
From www.scribd.com
Salt 2 Lead Nitrate PDF Nitrate Sulfuric Acid Lead Nitrate Relative Formula Mass Enter the molecular formula of the substance. Compute mass of each element. This site explains how to. Track your food intake, exercise, sleep and meditation for. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. Molar mass of pb(no3)2 = 331.2098 g/mol. [2] the molar mass of oxygen is 15.999 g/mol. (a r of mg =.. Lead Nitrate Relative Formula Mass.
From www.teachoo.com
MCQ Reema took 5ml of Lead Nitrate solution in a beaker and added ap Lead Nitrate Relative Formula Mass [1] the molar mass of nitrogen is 14.007 g/mol. [2] the molar mass of oxygen is 15.999 g/mol. (a r of mg =. Compute mass of each element. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. Enter the molecular formula of the. Lead Nitrate Relative Formula Mass.
From www.youtube.com
How to write Molecular formula of lead nitrate Chemical formula of Lead Nitrate Relative Formula Mass Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. This site explains how to. It will calculate the total mass along with the elemental composition and mass of each. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. [2] the molar mass of oxygen is 15.999 g/mol. Molar mass of pb(no3)2 =. Lead Nitrate Relative Formula Mass.
From molekula.com
Purchase Lead (II) nitrate [10099748] online • Catalog • Molekula Group Lead Nitrate Relative Formula Mass Molar mass of pb(no3)2 = 331.2098 g/mol. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass. [3] now, to calculate the molar mass of pb. Track your food intake, exercise, sleep and meditation for. It will calculate the total mass along with the elemental composition and mass. Lead Nitrate Relative Formula Mass.
From www.youtube.com
WHAT HAPPENS WHEN LEAD NITRATE (Pb(NO3)2 ) IS HEATED ? CLASS10 Lead Nitrate Relative Formula Mass Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass. Convert grams lead nitrate to moles. [3] now, to calculate the molar mass of pb. Molar mass of pb(no3)2 = 331.2098 g/mol. Compute mass of each element. [2] the molar mass of oxygen is 15.999 g/mol. Enter the. Lead Nitrate Relative Formula Mass.
From www.coursehero.com
[Solved] Lead (II) nitrate volume (ml)=1.95, Molarity (m)= 0.268 Lead Nitrate Relative Formula Mass Enter the molecular formula of the substance. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass. [3] now, to calculate the molar mass of pb. Convert grams lead nitrate to moles. [1] the molar mass of nitrogen is 14.007 g/mol. This site explains how to. Track your. Lead Nitrate Relative Formula Mass.
From www.studocu.com
LEAD Nitrate 1 EXPERIMENT NO DATE LEAD NITRATE AIM To analyse Lead Nitrate Relative Formula Mass Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. Convert grams lead nitrate to moles. [3] now, to calculate the molar mass of pb. Track your food intake, exercise, sleep and meditation for. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. Multiply the number of atoms by the atomic weight of. Lead Nitrate Relative Formula Mass.
From www.numerade.com
SOLVEDWhen a solution of lead(II) nitrate is mixed with a solution of Lead Nitrate Relative Formula Mass (a r of mg =. Compute mass of each element. Molar mass of pb(no3)2 = 331.2098 g/mol. [1] the molar mass of nitrogen is 14.007 g/mol. [2] the molar mass of oxygen is 15.999 g/mol. Enter the molecular formula of the substance. It will calculate the total mass along with the elemental composition and mass of each. [3] now, to. Lead Nitrate Relative Formula Mass.
From askfilo.com
Chemical formula of lead nitrate Filo Lead Nitrate Relative Formula Mass [2] the molar mass of oxygen is 15.999 g/mol. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. Convert grams lead nitrate to moles. (a r of mg =. Enter the molecular formula of the substance. [1] the molar mass of nitrogen is. Lead Nitrate Relative Formula Mass.
From yazmingokefoster.blogspot.com
Particle Diagram of Lead Nitrate and Potassium Iodide Lead Nitrate Relative Formula Mass Compute mass of each element. (a r of mg =. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. Enter the molecular formula of the substance. This site explains how to. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. Multiply the number of atoms by the atomic weight of each element. Lead Nitrate Relative Formula Mass.
From www.youtube.com
Pb(NO3)2 Molar Mass / Molecular Weight YouTube Lead Nitrate Relative Formula Mass Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. Molar mass of pb(no3)2 = 331.2098 g/mol. [2] the molar mass of oxygen is 15.999 g/mol. This site explains how to. [1] the molar mass of nitrogen is 14.007 g/mol. Multiply the number of atoms by the atomic weight of each element found in steps 1 and. Lead Nitrate Relative Formula Mass.
From lab.honeywell.com
Lead(II) nitrate 228621 Honeywell Research Chemicals Lead Nitrate Relative Formula Mass Molar mass of pb(no3)2 = 331.2098 g/mol. Convert grams lead nitrate to moles. It will calculate the total mass along with the elemental composition and mass of each. [2] the molar mass of oxygen is 15.999 g/mol. This site explains how to. [3] now, to calculate the molar mass of pb. Multiply the number of atoms by the atomic weight. Lead Nitrate Relative Formula Mass.
From www.toppr.com
If the relative molecular mass of ammonium nitrate is 80, the Lead Nitrate Relative Formula Mass [3] now, to calculate the molar mass of pb. This site explains how to. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. (a r of mg =. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. Multiply the number of atoms by the atomic weight of each element found in steps. Lead Nitrate Relative Formula Mass.
From www.youtube.com
Is Pb(NO3)2, Lead (II) nitrate, Ionic or Covalent? YouTube Lead Nitrate Relative Formula Mass Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. [2] the molar mass of oxygen is 15.999 g/mol. Track your food intake, exercise, sleep and meditation for. It will calculate the total mass along with the elemental composition and mass of each. Enter the molecular formula of the substance. This site explains how to. (a r. Lead Nitrate Relative Formula Mass.
From www.youtube.com
Lead II Nitrate Preparation and Properties YouTube Lead Nitrate Relative Formula Mass [2] the molar mass of oxygen is 15.999 g/mol. (a r of mg =. [3] now, to calculate the molar mass of pb. Compute mass of each element. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. The molar mass of lead is 207.2 g/mol. Molar mass of pb(no3)2 = 331.2098 g/mol. Convert grams lead nitrate. Lead Nitrate Relative Formula Mass.
From testbook.com
Lead (II) Nitrate Formula Structure, Preparation, & Uses Lead Nitrate Relative Formula Mass [3] now, to calculate the molar mass of pb. [1] the molar mass of nitrogen is 14.007 g/mol. Molar mass of pb(no3)2 = 331.2098 g/mol. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. (a r of mg =. The molar mass of lead is 207.2 g/mol. Track your food intake, exercise, sleep and meditation for.. Lead Nitrate Relative Formula Mass.
From pharmabeej.com
Preparation Of 0.1M Lead Nitrate In Pharma Pharmabeej Lead Nitrate Relative Formula Mass [3] now, to calculate the molar mass of pb. It will calculate the total mass along with the elemental composition and mass of each. (a r of mg =. Molar mass of pb(no3)2 = 331.2098 g/mol. [2] the molar mass of oxygen is 15.999 g/mol. Multiply the number of atoms by the atomic weight of each element found in steps. Lead Nitrate Relative Formula Mass.
From www.youtube.com
How to calculate the molar mass of calcium nitrate YouTube Lead Nitrate Relative Formula Mass Track your food intake, exercise, sleep and meditation for. (a r of mg =. Enter the molecular formula of the substance. Molar mass of lead (ii) nitrate (pb (no 3) 2) is 331.2098 g/mol. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. [2] the molar mass of oxygen is 15.999 g/mol. The molar mass of. Lead Nitrate Relative Formula Mass.
From www.scribd.com
Lead Nitrate PDF Salt (Chemistry) Sulfuric Acid Lead Nitrate Relative Formula Mass It will calculate the total mass along with the elemental composition and mass of each. The molar mass of lead is 207.2 g/mol. This site explains how to. [2] the molar mass of oxygen is 15.999 g/mol. Convert grams lead nitrate to moles. Track your food intake, exercise, sleep and meditation for. Multiply the number of atoms by the atomic. Lead Nitrate Relative Formula Mass.
From www.sigmaaldrich.id
LEAD(II) NITRATE, 99+, A.C.S. REAGENT Merck Life Science Indonesia Lead Nitrate Relative Formula Mass [1] the molar mass of nitrogen is 14.007 g/mol. The molar mass of lead is 207.2 g/mol. [2] the molar mass of oxygen is 15.999 g/mol. (a r of mg =. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass. Calculate the relative formula mass, m r,. Lead Nitrate Relative Formula Mass.
From slideplayer.com
Lead Nitrate Suppression of Staph. E Biofilm Formation ppt download Lead Nitrate Relative Formula Mass Compute mass of each element. This site explains how to. Molar mass of pb(no3)2 = 331.2098 g/mol. Calculate the relative formula mass, m r, of magnesium nitrate, mg(no 3) 2. [2] the molar mass of oxygen is 15.999 g/mol. [3] now, to calculate the molar mass of pb. Convert grams lead nitrate to moles. The molar mass of lead is. Lead Nitrate Relative Formula Mass.
From www.coursehero.com
[Solved] Lead(II) nitrate reacts with potassium chromate to form lead Lead Nitrate Relative Formula Mass [2] the molar mass of oxygen is 15.999 g/mol. This site explains how to. Molar mass of pb(no3)2 = 331.2098 g/mol. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass. (a r of mg =. [3] now, to calculate the molar mass of pb. Track your food. Lead Nitrate Relative Formula Mass.