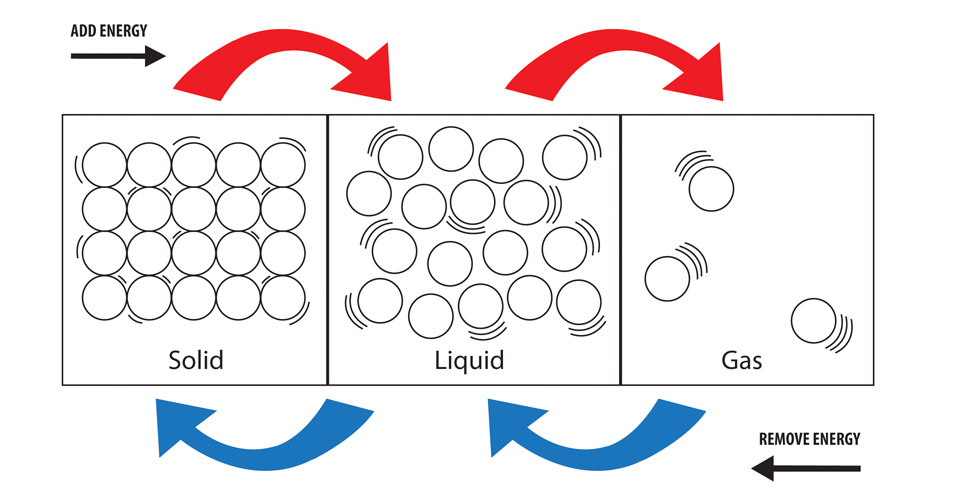
Solid To Liquid Energy Increase Or Decrease . exothermic processes go from high energy to low energy: Gas to liquid to solid. The conversion of a solid to a. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. phase transitions play an important theoretical and practical role in the study of heat flow. In melting (or “ fusion. This occurs when solids absorb enough energy to completely. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. When a liquid is converted to a solid, this change of state is referred to as. on a molecular level, the intermolecular forces between the water molecules are decreasing. The heat is providing enough. the process in which solids directly change to gases is known as sublimation. A very common phase change is between liquid and solids.
from socratic.org
the process in which solids directly change to gases is known as sublimation. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. The heat is providing enough. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. exothermic processes go from high energy to low energy: phase transitions play an important theoretical and practical role in the study of heat flow. A very common phase change is between liquid and solids. on a molecular level, the intermolecular forces between the water molecules are decreasing. The conversion of a solid to a. Gas to liquid to solid.
What happens to the energy of its molecules as ice melts into
Solid To Liquid Energy Increase Or Decrease exothermic processes go from high energy to low energy: The conversion of a solid to a. When a liquid is converted to a solid, this change of state is referred to as. Gas to liquid to solid. The heat is providing enough. In melting (or “ fusion. the process in which solids directly change to gases is known as sublimation. A very common phase change is between liquid and solids. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. on a molecular level, the intermolecular forces between the water molecules are decreasing. exothermic processes go from high energy to low energy: at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. This occurs when solids absorb enough energy to completely. phase transitions play an important theoretical and practical role in the study of heat flow.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid To Liquid Energy Increase Or Decrease When a liquid is converted to a solid, this change of state is referred to as. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. exothermic processes go from high energy to low energy: The heat is providing enough. The conversion of a solid to. Solid To Liquid Energy Increase Or Decrease.
From courses.lumenlearning.com
Phase Diagrams Chemistry Solid To Liquid Energy Increase Or Decrease phase transitions play an important theoretical and practical role in the study of heat flow. The heat is providing enough. the process in which solids directly change to gases is known as sublimation. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. This occurs when. Solid To Liquid Energy Increase Or Decrease.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Solid To Liquid Energy Increase Or Decrease In melting (or “ fusion. This occurs when solids absorb enough energy to completely. The conversion of a solid to a. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. on a molecular level, the intermolecular forces between the water molecules are decreasing. When a. Solid To Liquid Energy Increase Or Decrease.
From socratic.org
What happens to the energy of its molecules as ice melts into Solid To Liquid Energy Increase Or Decrease In melting (or “ fusion. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. The heat is providing enough. Gas to liquid to solid. the process in which solids directly change to gases is known as sublimation. The conversion of a solid to a. . Solid To Liquid Energy Increase Or Decrease.
From www.worldwisetutoring.com
Heating and Cooling Curves Solid To Liquid Energy Increase Or Decrease the process in which solids directly change to gases is known as sublimation. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. phase transitions play an important theoretical and practical role in the study of heat flow. This occurs when solids absorb enough energy. Solid To Liquid Energy Increase Or Decrease.
From wisc.pb.unizin.org
Explaining Solubility and Surface Tension through IMFs (M10Q4) UW Solid To Liquid Energy Increase Or Decrease the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. exothermic processes go from high energy to low energy: phase transitions play an important theoretical and practical role in the study of heat flow. This occurs when solids absorb enough energy to completely. The heat is. Solid To Liquid Energy Increase Or Decrease.
From www.britannica.com
phase Definition & Facts Britannica Solid To Liquid Energy Increase Or Decrease exothermic processes go from high energy to low energy: the process in which solids directly change to gases is known as sublimation. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. The conversion of a solid to a. When a liquid is converted to. Solid To Liquid Energy Increase Or Decrease.
From kids.britannica.com
solid Students Britannica Kids Homework Help Solid To Liquid Energy Increase Or Decrease the process in which solids directly change to gases is known as sublimation. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. This. Solid To Liquid Energy Increase Or Decrease.
From courses.lumenlearning.com
8.2 Solids and Liquids The Basics of General, Organic, and Biological Solid To Liquid Energy Increase Or Decrease When a liquid is converted to a solid, this change of state is referred to as. the process in which solids directly change to gases is known as sublimation. A very common phase change is between liquid and solids. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in. Solid To Liquid Energy Increase Or Decrease.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Solid To Liquid Energy Increase Or Decrease phase transitions play an important theoretical and practical role in the study of heat flow. A very common phase change is between liquid and solids. on a molecular level, the intermolecular forces between the water molecules are decreasing. This occurs when solids absorb enough energy to completely. The heat is providing enough. the process in which solids. Solid To Liquid Energy Increase Or Decrease.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solid To Liquid Energy Increase Or Decrease phase transitions play an important theoretical and practical role in the study of heat flow. This occurs when solids absorb enough energy to completely. A very common phase change is between liquid and solids. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. Gas to. Solid To Liquid Energy Increase Or Decrease.
From courses.lumenlearning.com
Phase Changes Physics Solid To Liquid Energy Increase Or Decrease The heat is providing enough. Gas to liquid to solid. When a liquid is converted to a solid, this change of state is referred to as. In melting (or “ fusion. A very common phase change is between liquid and solids. phase transitions play an important theoretical and practical role in the study of heat flow. the process. Solid To Liquid Energy Increase Or Decrease.
From www.learnatnoon.com
The Molecular Differences Between Solids, Liquid and Gases Solid To Liquid Energy Increase Or Decrease The heat is providing enough. phase transitions play an important theoretical and practical role in the study of heat flow. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. exothermic processes go from high energy to low energy: on a molecular level, the intermolecular. Solid To Liquid Energy Increase Or Decrease.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Solid To Liquid Energy Increase Or Decrease the process in which solids directly change to gases is known as sublimation. phase transitions play an important theoretical and practical role in the study of heat flow. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. exothermic processes go from high energy. Solid To Liquid Energy Increase Or Decrease.
From www.yaclass.in
Effect of heat on solid, liquid and gases — lesson. Science State Board Solid To Liquid Energy Increase Or Decrease The heat is providing enough. In melting (or “ fusion. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. When a liquid is converted to a solid, this change of state is referred to as. A very common phase change is between liquid and solids. This. Solid To Liquid Energy Increase Or Decrease.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Solid To Liquid Energy Increase Or Decrease This occurs when solids absorb enough energy to completely. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. phase transitions play an important theoretical and practical role in the study of heat flow. Gas to liquid to solid. exothermic processes go from high energy to. Solid To Liquid Energy Increase Or Decrease.
From lavelle.chem.ucla.edu
melting CHEMISTRY COMMUNITY Solid To Liquid Energy Increase Or Decrease When a liquid is converted to a solid, this change of state is referred to as. The conversion of a solid to a. on a molecular level, the intermolecular forces between the water molecules are decreasing. Gas to liquid to solid. The heat is providing enough. the term is most commonly used to describe transitions between solid, liquid. Solid To Liquid Energy Increase Or Decrease.
From www.elevise.co.uk
G O GS 1.1 Elevise Solid To Liquid Energy Increase Or Decrease The conversion of a solid to a. Gas to liquid to solid. phase transitions play an important theoretical and practical role in the study of heat flow. on a molecular level, the intermolecular forces between the water molecules are decreasing. When a liquid is converted to a solid, this change of state is referred to as. exothermic. Solid To Liquid Energy Increase Or Decrease.
From www.ase.org.uk
Solids, liquids and gases Solid To Liquid Energy Increase Or Decrease When a liquid is converted to a solid, this change of state is referred to as. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. A very common phase change is between liquid and solids. phase transitions play an important theoretical and practical role in. Solid To Liquid Energy Increase Or Decrease.
From courses.lumenlearning.com
Phase Change and Latent Heat Physics Solid To Liquid Energy Increase Or Decrease Gas to liquid to solid. This occurs when solids absorb enough energy to completely. A very common phase change is between liquid and solids. In melting (or “ fusion. phase transitions play an important theoretical and practical role in the study of heat flow. the term is most commonly used to describe transitions between solid, liquid and gaseous. Solid To Liquid Energy Increase Or Decrease.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Solid To Liquid Energy Increase Or Decrease Gas to liquid to solid. In melting (or “ fusion. phase transitions play an important theoretical and practical role in the study of heat flow. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. A very common phase change is between liquid and solids. The heat. Solid To Liquid Energy Increase Or Decrease.
From bleckinger9sci.weebly.com
2. Chemistry Bleckinger Year 9 Science Solid To Liquid Energy Increase Or Decrease at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. phase transitions play an important theoretical and practical role in the study of heat. Solid To Liquid Energy Increase Or Decrease.
From jackwestin.com
Second Law Concept Of Entropy Energy Changes In Chemical Reactions Solid To Liquid Energy Increase Or Decrease When a liquid is converted to a solid, this change of state is referred to as. The conversion of a solid to a. exothermic processes go from high energy to low energy: phase transitions play an important theoretical and practical role in the study of heat flow. Gas to liquid to solid. the term is most commonly. Solid To Liquid Energy Increase Or Decrease.
From courses.lumenlearning.com
Entropy Chemistry for Majors Solid To Liquid Energy Increase Or Decrease A very common phase change is between liquid and solids. This occurs when solids absorb enough energy to completely. exothermic processes go from high energy to low energy: the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. In melting (or “ fusion. phase transitions play. Solid To Liquid Energy Increase Or Decrease.
From courses.lumenlearning.com
Solid to Gas Phase Transition Introduction to Chemistry Solid To Liquid Energy Increase Or Decrease Gas to liquid to solid. This occurs when solids absorb enough energy to completely. the process in which solids directly change to gases is known as sublimation. on a molecular level, the intermolecular forces between the water molecules are decreasing. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter. Solid To Liquid Energy Increase Or Decrease.
From socratic.org
For the three states of matter (solid, liquid, and gas) there are six Solid To Liquid Energy Increase Or Decrease A very common phase change is between liquid and solids. the process in which solids directly change to gases is known as sublimation. The heat is providing enough. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. Gas to liquid to solid. The conversion of. Solid To Liquid Energy Increase Or Decrease.
From www.elevise.co.uk
P3 E) States of Matter AQA Combined Science Trilogy Elevise Solid To Liquid Energy Increase Or Decrease This occurs when solids absorb enough energy to completely. exothermic processes go from high energy to low energy: A very common phase change is between liquid and solids. The conversion of a solid to a. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. When a. Solid To Liquid Energy Increase Or Decrease.
From www.snexplores.org
Explainer What are the different states of matter? Solid To Liquid Energy Increase Or Decrease exothermic processes go from high energy to low energy: In melting (or “ fusion. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. The heat is providing enough. A very common phase change is between liquid and solids. When a liquid is converted to a solid,. Solid To Liquid Energy Increase Or Decrease.
From www.britannica.com
phase Definition & Facts Britannica Solid To Liquid Energy Increase Or Decrease at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. In melting (or “ fusion. the process in which solids directly change to gases is known as sublimation. exothermic processes go from high energy to low energy: The conversion of a solid to a. . Solid To Liquid Energy Increase Or Decrease.
From courses.lumenlearning.com
Measuring Entropy and Entropy Changes Introductory Chemistry Solid To Liquid Energy Increase Or Decrease phase transitions play an important theoretical and practical role in the study of heat flow. on a molecular level, the intermolecular forces between the water molecules are decreasing. the process in which solids directly change to gases is known as sublimation. Gas to liquid to solid. The heat is providing enough. When a liquid is converted to. Solid To Liquid Energy Increase Or Decrease.
From byjus.com
Draw a sketch to show the arrangement of particles in solid, liquid and Solid To Liquid Energy Increase Or Decrease A very common phase change is between liquid and solids. This occurs when solids absorb enough energy to completely. The heat is providing enough. phase transitions play an important theoretical and practical role in the study of heat flow. Gas to liquid to solid. the process in which solids directly change to gases is known as sublimation. In. Solid To Liquid Energy Increase Or Decrease.
From philschatz.com
Intermolecular Forces · Chemistry Solid To Liquid Energy Increase Or Decrease exothermic processes go from high energy to low energy: phase transitions play an important theoretical and practical role in the study of heat flow. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. Gas to liquid to solid. the process in which solids directly. Solid To Liquid Energy Increase Or Decrease.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Solid To Liquid Energy Increase Or Decrease exothermic processes go from high energy to low energy: phase transitions play an important theoretical and practical role in the study of heat flow. the term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases,. at some point, the added energy becomes large enough to partially. Solid To Liquid Energy Increase Or Decrease.
From socratic.org
Question 15820 Socratic Solid To Liquid Energy Increase Or Decrease on a molecular level, the intermolecular forces between the water molecules are decreasing. In melting (or “ fusion. exothermic processes go from high energy to low energy: the process in which solids directly change to gases is known as sublimation. Gas to liquid to solid. the term is most commonly used to describe transitions between solid,. Solid To Liquid Energy Increase Or Decrease.
From ch301.cm.utexas.edu
heating curve Solid To Liquid Energy Increase Or Decrease A very common phase change is between liquid and solids. Gas to liquid to solid. at some point, the added energy becomes large enough to partially overcome the forces holding the molecules or ions of the solid. When a liquid is converted to a solid, this change of state is referred to as. phase transitions play an important. Solid To Liquid Energy Increase Or Decrease.