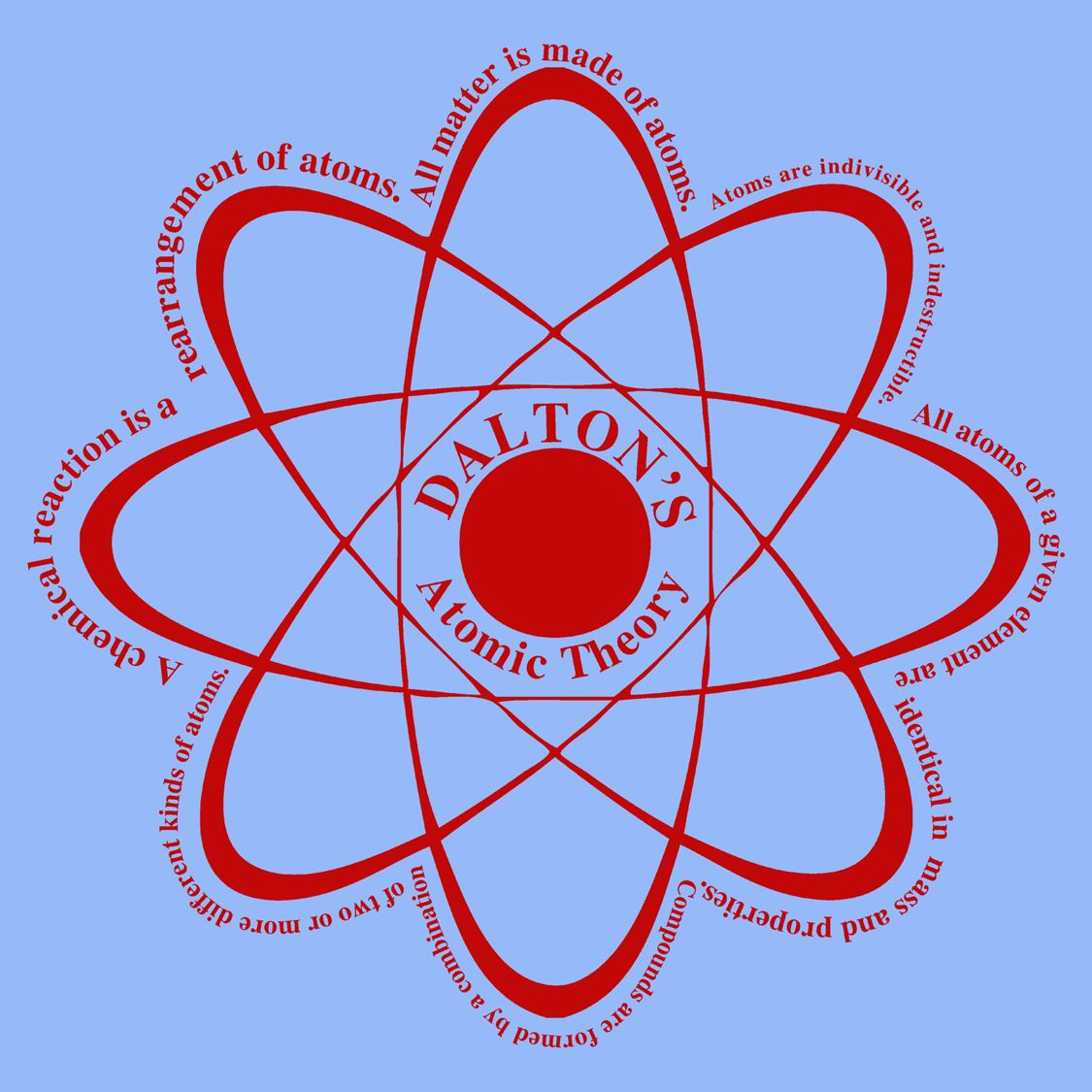
What Are The 5 Parts Of John Dalton's Atomic Theory . The properties of all the atoms of a. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. John dalton, a british school teacher, published his theory about atoms in 1808. Postulates of dalton’s atomic theory. The matter is made up of indivisible particles known as atoms. Dalton’s atomic theory and 5 postulates. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. There are two main merits of dalton’s atomic theory. Postulates of dalton's atomic theory. (a) dalton’s atomic theory successfully explained the law of.
from www.w3schools.blog
(a) dalton’s atomic theory successfully explained the law of. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. There are two main merits of dalton’s atomic theory. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. The matter is made up of indivisible particles known as atoms. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. Dalton’s atomic theory and 5 postulates. John dalton, a british school teacher, published his theory about atoms in 1808. Postulates of dalton's atomic theory. The properties of all the atoms of a.
Dalton’s Atomic Theory W3schools
What Are The 5 Parts Of John Dalton's Atomic Theory The matter is made up of indivisible particles known as atoms. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. John dalton, a british school teacher, published his theory about atoms in 1808. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. The matter is made up of indivisible particles known as atoms. Postulates of dalton’s atomic theory. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. Postulates of dalton's atomic theory. There are two main merits of dalton’s atomic theory. The properties of all the atoms of a. (a) dalton’s atomic theory successfully explained the law of. Dalton’s atomic theory and 5 postulates. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called.
From hubpages.com
Atoms and Atomic Structure HubPages What Are The 5 Parts Of John Dalton's Atomic Theory Dalton’s atomic theory and 5 postulates. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. The matter is made up of indivisible particles known as atoms. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.youtube.com
Dalton's Atomic Theory YouTube What Are The 5 Parts Of John Dalton's Atomic Theory Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. (a) dalton’s atomic theory successfully explained the law of. The properties of all the atoms of a. Postulates of dalton’s atomic theory. There are two main merits of dalton’s atomic theory. Dalton's atomic theory (1804) from his own experiments. What Are The 5 Parts Of John Dalton's Atomic Theory.
From uwaterloo.ca
Know the limit and teach within it! Part 3 Evolution and revolution What Are The 5 Parts Of John Dalton's Atomic Theory Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. The properties of all the atoms of a. (a) dalton’s atomic theory successfully explained the law of. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.britannica.com
John Dalton's atomic theory explained Britannica What Are The 5 Parts Of John Dalton's Atomic Theory Dalton’s atomic theory and 5 postulates. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. Postulates of dalton's atomic theory. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. There are two main merits of dalton’s atomic theory.. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.slideshare.net
Dalton's Atomic Theory What Are The 5 Parts Of John Dalton's Atomic Theory Dalton’s atomic theory and 5 postulates. (a) dalton’s atomic theory successfully explained the law of. The properties of all the atoms of a. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. Postulates of dalton's atomic theory. Dalton's atomic theory is the first scientific. What Are The 5 Parts Of John Dalton's Atomic Theory.
From ar.inspiredpencil.com
Daltons Atomic Model Labeled What Are The 5 Parts Of John Dalton's Atomic Theory The properties of all the atoms of a. John dalton, a british school teacher, published his theory about atoms in 1808. Postulates of dalton’s atomic theory. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. The matter is made up of indivisible particles. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.homeworkjoy.com
What Are the 5 Postulates of Dalton's Atomic Theory What Are The 5 Parts Of John Dalton's Atomic Theory Postulates of dalton’s atomic theory. Dalton’s atomic theory and 5 postulates. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. The properties of all the atoms of a. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.slideserve.com
PPT John Dalton’s Atomic Theory 1803 PowerPoint Presentation, free What Are The 5 Parts Of John Dalton's Atomic Theory The properties of all the atoms of a. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. John dalton, a british school teacher, published his theory about atoms in 1808. Dalton’s atomic theory and 5 postulates. Dalton’s atomic theory was a scientific theory. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.w3schools.blog
Dalton’s Atomic Theory W3schools What Are The 5 Parts Of John Dalton's Atomic Theory Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. The matter is made up of indivisible particles known as atoms. John dalton, a british school teacher, published his theory about atoms in 1808. The properties of all the atoms of a. Dalton’s atomic theory was a scientific theory. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.worksheetsplanet.com
Dalton's Atomic Theory What Are The 5 Parts Of John Dalton's Atomic Theory Postulates of dalton's atomic theory. There are two main merits of dalton’s atomic theory. (a) dalton’s atomic theory successfully explained the law of. John dalton, a british school teacher, published his theory about atoms in 1808. Postulates of dalton’s atomic theory. The matter is made up of indivisible particles known as atoms. Dalton's atomic theory (1804) from his own experiments. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.slideserve.com
PPT Chapter 4 Atoms PowerPoint Presentation, free download ID5528711 What Are The 5 Parts Of John Dalton's Atomic Theory The properties of all the atoms of a. Postulates of dalton’s atomic theory. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. There are two main merits of dalton’s atomic theory. John dalton, a british school teacher, published his theory about atoms in 1808.. What Are The 5 Parts Of John Dalton's Atomic Theory.
From joannakruwmendoza.blogspot.com
John Dalton Atomic Model JoannakruwMendoza What Are The 5 Parts Of John Dalton's Atomic Theory The properties of all the atoms of a. (a) dalton’s atomic theory successfully explained the law of. John dalton, a british school teacher, published his theory about atoms in 1808. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. Dalton's atomic theory (1804) from his own experiments and observations, as. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.scienceabc.com
What Is The Dalton Atomic Model? What Are The 5 Parts Of John Dalton's Atomic Theory The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. Dalton’s atomic theory and 5 postulates. There are two main merits of dalton’s atomic theory. The. What Are The 5 Parts Of John Dalton's Atomic Theory.
From noticiasmodelo.blogspot.com
John Dalton Atomic Model Date Of Discovery Noticias Modelo What Are The 5 Parts Of John Dalton's Atomic Theory Postulates of dalton's atomic theory. (a) dalton’s atomic theory successfully explained the law of. Dalton’s atomic theory and 5 postulates. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. Postulates of dalton’s atomic theory. The five postulates below summarize dalton’s atomic theory… #1 —. What Are The 5 Parts Of John Dalton's Atomic Theory.
From atomictheorysquad.weebly.com
Dalton Atomic Theory What Are The 5 Parts Of John Dalton's Atomic Theory The matter is made up of indivisible particles known as atoms. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. (a) dalton’s atomic theory successfully explained the law of. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.youtube.com
ATOMIC THEORY2 [JOHN DALTON] YouTube What Are The 5 Parts Of John Dalton's Atomic Theory Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. John dalton, a british school teacher, published his theory about atoms in 1808. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom.. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.slideserve.com
PPT Dalton’s Atomic Theory (1808) PowerPoint Presentation, free What Are The 5 Parts Of John Dalton's Atomic Theory There are two main merits of dalton’s atomic theory. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. The matter is made up of indivisible particles known as atoms. John dalton, a british school teacher, published his theory about atoms in 1808. Dalton's atomic theory (1804) from his own experiments. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.expii.com
Dalton's Atomic Theory — Overview & Modern Application Expii What Are The 5 Parts Of John Dalton's Atomic Theory Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. Dalton’s atomic theory and 5 postulates. There are two main merits of dalton’s. What Are The 5 Parts Of John Dalton's Atomic Theory.
From ar.inspiredpencil.com
Daltons Atomic Model Labeled What Are The 5 Parts Of John Dalton's Atomic Theory The matter is made up of indivisible particles known as atoms. John dalton, a british school teacher, published his theory about atoms in 1808. The properties of all the atoms of a. Dalton’s atomic theory and 5 postulates. There are two main merits of dalton’s atomic theory. (a) dalton’s atomic theory successfully explained the law of. Dalton's atomic theory (1804). What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.slideserve.com
PPT John Daltons Atomic Theory PowerPoint Presentation, free download What Are The 5 Parts Of John Dalton's Atomic Theory Postulates of dalton’s atomic theory. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. Postulates of dalton's atomic theory. (a) dalton’s atomic. What Are The 5 Parts Of John Dalton's Atomic Theory.
From mungfali.com
John Dalton Theory Model What Are The 5 Parts Of John Dalton's Atomic Theory The matter is made up of indivisible particles known as atoms. Postulates of dalton's atomic theory. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. Postulates of dalton’s atomic theory. Dalton's atomic theory (1804) from his own experiments and observations, as well as the. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.slideserve.com
PPT Chapter 2 Matter is Made up of Atoms PowerPoint Presentation What Are The 5 Parts Of John Dalton's Atomic Theory Dalton’s atomic theory and 5 postulates. The properties of all the atoms of a. Postulates of dalton’s atomic theory. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. Postulates of dalton's atomic theory. John dalton, a british school teacher, published his theory about. What Are The 5 Parts Of John Dalton's Atomic Theory.
From chemistnotes.com
Dalton's Atomic Theory Postulates and limitations Chemistry Notes What Are The 5 Parts Of John Dalton's Atomic Theory Postulates of dalton’s atomic theory. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. Dalton’s atomic theory and 5 postulates. There are two main. What Are The 5 Parts Of John Dalton's Atomic Theory.
From quiztouchingly.z21.web.core.windows.net
Who Proposed The Atomic Theory What Are The 5 Parts Of John Dalton's Atomic Theory There are two main merits of dalton’s atomic theory. John dalton, a british school teacher, published his theory about atoms in 1808. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his. What Are The 5 Parts Of John Dalton's Atomic Theory.
From scienceworldfactors.blogspot.com
Concepts of atom What Are The 5 Parts Of John Dalton's Atomic Theory Postulates of dalton’s atomic theory. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. The five postulates below. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.universetoday.com
atomic theory Archives Universe Today What Are The 5 Parts Of John Dalton's Atomic Theory Dalton’s atomic theory and 5 postulates. Postulates of dalton’s atomic theory. Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year. The matter is made up of indivisible particles known as atoms. Postulates of dalton's atomic theory. The properties of all the atoms of a.. What Are The 5 Parts Of John Dalton's Atomic Theory.
From ck12.org
Dalton's Atomic Theory CK12 Foundation What Are The 5 Parts Of John Dalton's Atomic Theory Dalton’s atomic theory and 5 postulates. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. Postulates of dalton’s atomic theory. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. The properties of all the atoms of a. John. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.youtube.com
Dalton's atomic theory rcfy chemistry YouTube What Are The 5 Parts Of John Dalton's Atomic Theory (a) dalton’s atomic theory successfully explained the law of. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. Dalton’s atomic theory and 5 postulates. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. The matter is made up. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.teachoo.com
Dalton's Atomic Theory Postulate, Limitations [Teachoo] What Are The 5 Parts Of John Dalton's Atomic Theory Postulates of dalton’s atomic theory. (a) dalton’s atomic theory successfully explained the law of. Dalton’s atomic theory and 5 postulates. The properties of all the atoms of a. The matter is made up of indivisible particles known as atoms. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. Dalton's atomic. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.sliderbase.com
Atomic Structure Presentation Chemistry What Are The 5 Parts Of John Dalton's Atomic Theory Postulates of dalton's atomic theory. There are two main merits of dalton’s atomic theory. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. Postulates of dalton’s atomic theory. John dalton, a british school teacher, published his theory about atoms in 1808. Dalton's atomic theory is the first scientific theory to. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.slideserve.com
PPT History of Atomic Theory PowerPoint Presentation, free download What Are The 5 Parts Of John Dalton's Atomic Theory There are two main merits of dalton’s atomic theory. Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. (a) dalton’s atomic theory successfully explained the law of. Postulates of dalton's atomic theory. Postulates of dalton’s atomic theory. Dalton's atomic theory (1804) from his own experiments and observations, as. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.pinterest.com
John Dalton Atomic Model What Are The 5 Parts Of John Dalton's Atomic Theory Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. There are two main merits of dalton’s atomic theory. Postulates of dalton’s atomic. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.jagranjosh.com
Dalton’s Atomic Theory Know in detail here What Are The 5 Parts Of John Dalton's Atomic Theory Dalton's atomic theory (1804) from his own experiments and observations, as well as the work of his peers, dalton proposed a new theory of the atom. Postulates of dalton's atomic theory. The properties of all the atoms of a. There are two main merits of dalton’s atomic theory. The five postulates below summarize dalton’s atomic theory… #1 — each element. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.slideserve.com
PPT Atomic History & Atomic Structure! PowerPoint Presentation ID What Are The 5 Parts Of John Dalton's Atomic Theory There are two main merits of dalton’s atomic theory. The five postulates below summarize dalton’s atomic theory… #1 — each element is composed of tiny indestructible particles called. Postulates of dalton’s atomic theory. The properties of all the atoms of a. Postulates of dalton's atomic theory. The matter is made up of indivisible particles known as atoms. Dalton's atomic theory. What Are The 5 Parts Of John Dalton's Atomic Theory.
From www.pinterest.com
Dalton's Atomic Theory. John dalton, Atomic theory, Chemistry education What Are The 5 Parts Of John Dalton's Atomic Theory Dalton's atomic theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom. Dalton’s atomic theory and 5 postulates. John dalton, a british school teacher, published his theory about atoms in 1808. Postulates of dalton’s atomic theory. Dalton's atomic theory (1804) from his own experiments and observations, as well as the work. What Are The 5 Parts Of John Dalton's Atomic Theory.