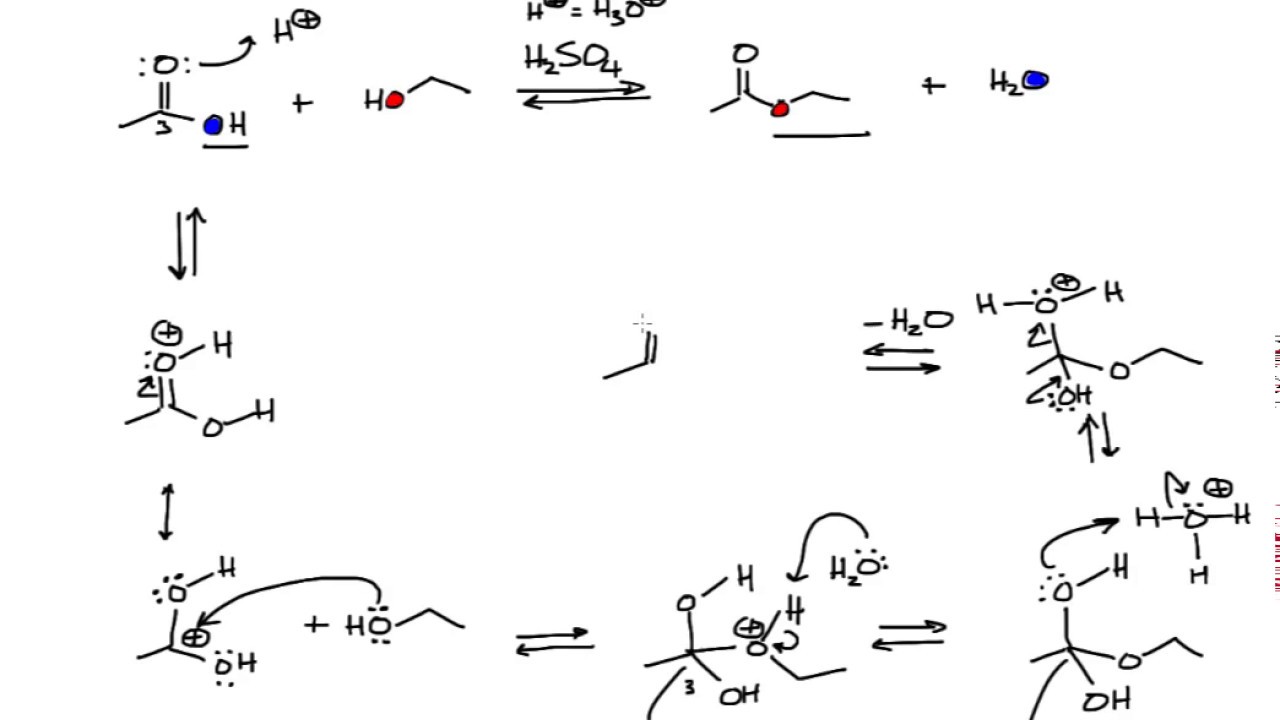
Basic Catalyst For Esterification Reaction . Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. The nucleophilic water reacts with. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Mechanism for acid catalyzed esterification. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. The methanol can act as a nucleophile to a.
from www.youtube.com
Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. The nucleophilic water reacts with. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. Mechanism for acid catalyzed esterification. The methanol can act as a nucleophile to a. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different.
Mechanism of the Fischer Esterification in Organic Chemistry YouTube
Basic Catalyst For Esterification Reaction Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Mechanism for acid catalyzed esterification. Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. The methanol can act as a nucleophile to a. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. The nucleophilic water reacts with.
From www.researchgate.net
Scheme 1 Overview of the catalytic esterification carried out with Basic Catalyst For Esterification Reaction The nucleophilic water reacts with. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. The methanol can act as a nucleophile to a. Esterification. Basic Catalyst For Esterification Reaction.
From www.chemistrylearner.com
Fischer Esterification Definition, Examples, and Mechanism Basic Catalyst For Esterification Reaction The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with. Basic Catalyst For Esterification Reaction.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Basic Catalyst For Esterification Reaction Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. The nucleophilic water reacts with. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a. Basic Catalyst For Esterification Reaction.
From www.masterorganicchemistry.com
Conversion of carboxylic acids to esters using acid and alcohols Basic Catalyst For Esterification Reaction The methanol can act as a nucleophile to a. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. Thus, carboxylic acids are converted directly into esters. Basic Catalyst For Esterification Reaction.
From www.researchgate.net
The mechanism of Fischer esterification catalysed by Brønsted acidic Basic Catalyst For Esterification Reaction Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. The methanol can act as a nucleophile to a. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Thus, carboxylic acids are converted directly into esters by. Basic Catalyst For Esterification Reaction.
From www.researchgate.net
Mechanism of the Steglich esterification. Download Scientific Diagram Basic Catalyst For Esterification Reaction The methanol can act as a nucleophile to a. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. The nucleophilic water reacts with. Thus, carboxylic acids are. Basic Catalyst For Esterification Reaction.
From www.researchgate.net
Mechanism of base catalyzed transesterification. Download Scientific Basic Catalyst For Esterification Reaction Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. This page looks in detail at the. Basic Catalyst For Esterification Reaction.
From www.youtube.com
Base Catalyzed Transesterification General Reaction YouTube Basic Catalyst For Esterification Reaction The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. The nucleophilic water reacts with. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. Thus, carboxylic acids are converted directly into esters by s n 2 reaction. Basic Catalyst For Esterification Reaction.
From www.researchgate.net
Mechanisms of ester hydrolysis under acid or base catalysis. Download Basic Catalyst For Esterification Reaction This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. The mechanism for the. Basic Catalyst For Esterification Reaction.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Basic Catalyst For Esterification Reaction The methanol can act as a nucleophile to a. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. Mechanism for acid catalyzed esterification. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. Mekala and goli. Basic Catalyst For Esterification Reaction.
From www.youtube.com
Fischer esterification from App Chemistry" Basic Reaction Basic Catalyst For Esterification Reaction Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. The nucleophilic water reacts with. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. Mechanism for. Basic Catalyst For Esterification Reaction.
From www.researchgate.net
Mechanism of heterogeneous acid catalyzed transesterification reaction Basic Catalyst For Esterification Reaction The nucleophilic water reacts with. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. The methanol can act as a nucleophile to a. Mechanism for acid catalyzed esterification. Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl. Basic Catalyst For Esterification Reaction.
From nrochemistry.com
Transesterification Reaction Basic Catalyst For Esterification Reaction Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. The methanol can act as a nucleophile to a. Mechanism for acid catalyzed esterification. The nucleophilic water reacts. Basic Catalyst For Esterification Reaction.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Basic Catalyst For Esterification Reaction Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Esterification is the process of combining an. Basic Catalyst For Esterification Reaction.
From www.youtube.com
Esterification Reaction Organic Chemistry YouTube Basic Catalyst For Esterification Reaction The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. The nucleophilic water reacts with.. Basic Catalyst For Esterification Reaction.
From www.researchgate.net
General mechanism for simultaneous esterification and... Download Basic Catalyst For Esterification Reaction The methanol can act as a nucleophile to a. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. The nucleophilic water reacts with. This page looks in detail at. Basic Catalyst For Esterification Reaction.
From www.researchgate.net
Esterification Mechanism with 0 Ni/Zeolite Catalyst. Esterification Basic Catalyst For Esterification Reaction The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. The methanol can act as a nucleophile to a. Thus, carboxylic acids are converted directly. Basic Catalyst For Esterification Reaction.
From chem.libretexts.org
21.3 Acid Catalyzed Esterification of Anhydrides Chemistry LibreTexts Basic Catalyst For Esterification Reaction Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Mechanism for acid catalyzed esterification. Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. The mechanism. Basic Catalyst For Esterification Reaction.
From www.youtube.com
Mechanism of the Fischer Esterification in Organic Chemistry YouTube Basic Catalyst For Esterification Reaction Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. The methanol can act as a nucleophile to a. Thus, carboxylic acids are converted directly into esters by. Basic Catalyst For Esterification Reaction.
From www.animalia-life.club
Esterification Mechanism Basic Catalyst For Esterification Reaction Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. This page looks in detail at the. Basic Catalyst For Esterification Reaction.
From www.masterorganicchemistry.com
Acid Catalysis of Organic Reactions Why It Works Basic Catalyst For Esterification Reaction This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase. Basic Catalyst For Esterification Reaction.
From www.youtube.com
Esterification Reaction of alcohol YouTube Basic Catalyst For Esterification Reaction The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. The nucleophilic water reacts with. Thus, carboxylic acids are converted directly into esters by s n 2 reaction. Basic Catalyst For Esterification Reaction.
From www.chemistrysteps.com
Fischer Esterification Chemistry Steps Basic Catalyst For Esterification Reaction Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. The nucleophilic water reacts with. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of. Basic Catalyst For Esterification Reaction.
From www.masterorganicchemistry.com
Transesterification Master Organic Chemistry Basic Catalyst For Esterification Reaction Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester (rcoor) and. The methanol can act as a nucleophile to a. Mechanism for acid catalyzed esterification. This page looks in detail at. Basic Catalyst For Esterification Reaction.
From www.researchgate.net
Fischer Esterification (A) general esterification reaction by the Basic Catalyst For Esterification Reaction This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Mechanism for acid catalyzed esterification. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of. Basic Catalyst For Esterification Reaction.
From chem.libretexts.org
Fischer Esterification Chemistry LibreTexts Basic Catalyst For Esterification Reaction The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Esterification is the process of combining an organic acid (rcooh) with an alcohol (roh) to form an ester. Basic Catalyst For Esterification Reaction.
From www.researchgate.net
Acidcatalyzed esterification. Reaction conditions and yields for Basic Catalyst For Esterification Reaction Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Mekala and goli performed simple. Basic Catalyst For Esterification Reaction.
From chem.libretexts.org
12.4 Esters Chemistry LibreTexts Basic Catalyst For Esterification Reaction Mechanism for acid catalyzed esterification. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. The mechanism. Basic Catalyst For Esterification Reaction.
From www.chemistrysteps.com
Fischer Esterification Chemistry Steps Basic Catalyst For Esterification Reaction Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase. Basic Catalyst For Esterification Reaction.
From www.masterorganicchemistry.com
Conversion of carboxylic acids to esters using acid and alcohols Basic Catalyst For Esterification Reaction This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Mechanism for acid catalyzed esterification. The methanol can act as a nucleophile to a. The mechanism for. Basic Catalyst For Esterification Reaction.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Basic Catalyst For Esterification Reaction The methanol can act as a nucleophile to a. The nucleophilic water reacts with. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. Mekala. Basic Catalyst For Esterification Reaction.
From www.youtube.com
Esterification Reaction Formation of Ester Organic Chemistry YouTube Basic Catalyst For Esterification Reaction The methanol can act as a nucleophile to a. Mechanism for acid catalyzed esterification. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. The. Basic Catalyst For Esterification Reaction.
From www.masterorganicchemistry.com
Transesterification Master Organic Chemistry Basic Catalyst For Esterification Reaction The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Thus, carboxylic acids are converted directly into esters by s n 2 reaction of a carboxylate ion with a primary alkyl halide or by fischer esterification of a carboxylic acid with an alcohol. This page looks in detail. Basic Catalyst For Esterification Reaction.
From animalia-life.club
Esterification Mechanism Basic Catalyst For Esterification Reaction Mechanism for acid catalyzed esterification. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. Mekala and goli performed simple bimolecular reaction between methanol and acetic acid with sulfuric acid as a catalyst at different. Esterification is the process of combining an organic acid (rcooh) with an. Basic Catalyst For Esterification Reaction.
From scienceinfo.com
Fischer Esterification Reaction Mechanism, Limitations Basic Catalyst For Esterification Reaction Mechanism for acid catalyzed esterification. This page looks in detail at the mechanism for the formation of esters from carboxylic acids and alcohols in the presence of concentrated. The nucleophilic water reacts with. The methanol can act as a nucleophile to a. The mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the. Basic Catalyst For Esterification Reaction.