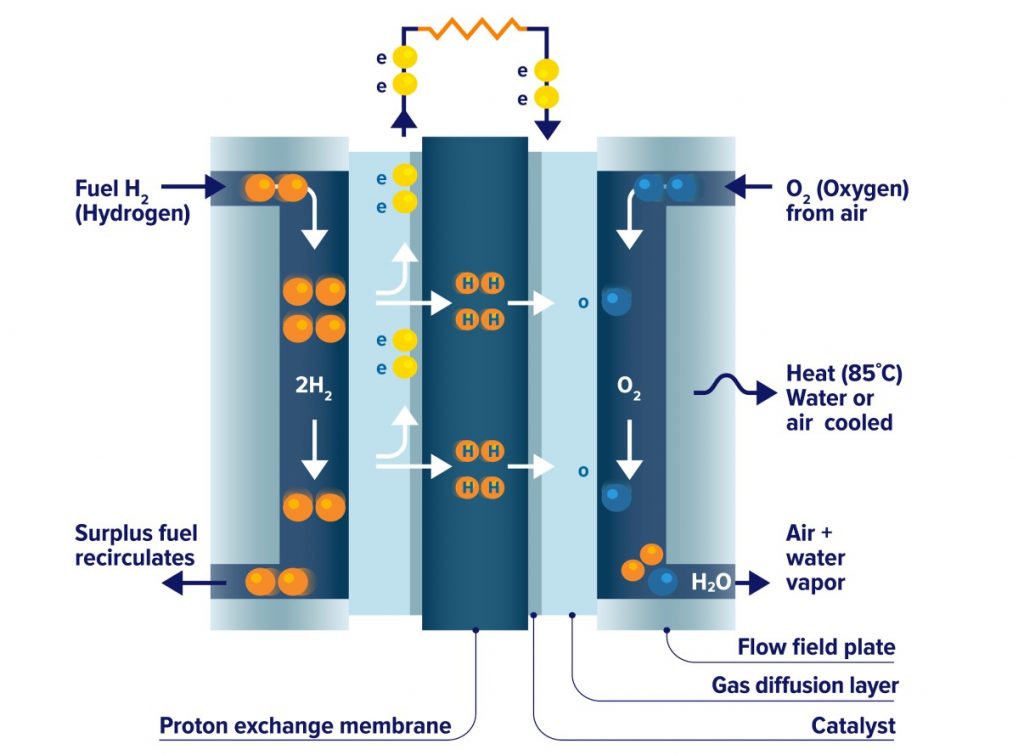
How Do Hydrogen Fuel Cells Work Gcse . →how does a fuel cell work? A chemical cell produces a voltage until one of the reactants is used up. The fuel is added to the cell and then there is a constant supply of oxygen. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. The fuel is oxidised electrochemically within the fuel cell. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. How does a hydrogen fuel cell work? Fuel cells work by using hydrogen and oxygen to produce an electrical current. A chemical cell produces a voltage until one of the reactants is used up. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is.
from climatebiz.com
How does a hydrogen fuel cell work? The fuel is oxidised electrochemically within the fuel cell. Fuel cells work by using hydrogen and oxygen to produce an electrical current. The fuel is added to the cell and then there is a constant supply of oxygen. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. →how does a fuel cell work? Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. Supplied by an external source of fuel (e.g hydrogen) and oxygen or air.
How Do Hydrogen Fuel Cells Work?
How Do Hydrogen Fuel Cells Work Gcse Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. The fuel is oxidised electrochemically within the fuel cell. A chemical cell produces a voltage until one of the reactants is used up. Fuel cells work by using hydrogen and oxygen to produce an electrical current. The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. How does a hydrogen fuel cell work? The fuel is added to the cell and then there is a constant supply of oxygen. →how does a fuel cell work? A chemical cell produces a voltage until one of the reactants is used up.
From elexexplorer.com
How Does Fuel Cell Work? Elex Explorer How Do Hydrogen Fuel Cells Work Gcse A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. A chemical cell produces a voltage until one of the reactants is used up. How does a hydrogen fuel cell work? Revision notes on. How Do Hydrogen Fuel Cells Work Gcse.
From www.shalom-education.com
Fuel Cells GCSE Chemistry Revision How Do Hydrogen Fuel Cells Work Gcse The fuel is added to the cell and then there is a constant supply of oxygen. Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. How does a hydrogen fuel cell work? The fuel is oxidised electrochemically within the fuel cell. A chemical cell produces a voltage until one of the reactants is used up. Revision. How Do Hydrogen Fuel Cells Work Gcse.
From www.vrogue.co
How Does A Hydrogen Fuel Cell Work Ecobatt Solutions vrogue.co How Do Hydrogen Fuel Cells Work Gcse Fuel cells work by using hydrogen and oxygen to produce an electrical current. How does a hydrogen fuel cell work? →how does a fuel cell work? The fuel is added to the cell and then there is a constant supply of oxygen. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at. How Do Hydrogen Fuel Cells Work Gcse.
From climatebiz.com
How Do Hydrogen Fuel Cells Work? How Do Hydrogen Fuel Cells Work Gcse A chemical cell produces a voltage until one of the reactants is used up. How does a hydrogen fuel cell work? A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. Fuel cells produce. How Do Hydrogen Fuel Cells Work Gcse.
From www.pinterest.com
FREE GCSE Chemistry (Science) Hydrogen Fuel Cells Practice Exam How Do Hydrogen Fuel Cells Work Gcse Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. →how does a fuel cell work? How does a hydrogen fuel cell work? The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. The fuel is oxidised electrochemically within the fuel cell. Fuel cells work by using hydrogen and oxygen. How Do Hydrogen Fuel Cells Work Gcse.
From mavink.com
Hydrogen Fuel Cell Process How Do Hydrogen Fuel Cells Work Gcse A chemical cell produces a voltage until one of the reactants is used up. The fuel is added to the cell and then there is a constant supply of oxygen. Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. Fuel cells work by using hydrogen and oxygen to produce an electrical current. The hydrogen is fed. How Do Hydrogen Fuel Cells Work Gcse.
From www.sukorun.com
Hydrogen Fuel Cells Q&A Part 1 Sukorun How Do Hydrogen Fuel Cells Work Gcse The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. Fuel cells work by using hydrogen and oxygen to produce an electrical current. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. How does a hydrogen fuel cell work? A chemical. How Do Hydrogen Fuel Cells Work Gcse.
From www.setra.com
What is a Hydrogen Fuel Cell and How Does it Work? How Do Hydrogen Fuel Cells Work Gcse How does a hydrogen fuel cell work? Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. Fuel cells work by using hydrogen and oxygen to produce an electrical current. A chemical cell produces a voltage until one of the reactants is used up. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written. How Do Hydrogen Fuel Cells Work Gcse.
From ar.inspiredpencil.com
How Hydrogen Fuel Cells Work How Do Hydrogen Fuel Cells Work Gcse A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. →how does a fuel cell work? A chemical cell produces a voltage until one of the reactants is used up. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The hydrogen is fed. How Do Hydrogen Fuel Cells Work Gcse.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room How Do Hydrogen Fuel Cells Work Gcse Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. How does a hydrogen fuel cell work? A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains.. How Do Hydrogen Fuel Cells Work Gcse.
From www.youtube.com
AQA GCSE Chemistry P1 Hydrogen Fuel Cells YouTube How Do Hydrogen Fuel Cells Work Gcse A chemical cell produces a voltage until one of the reactants is used up. The fuel is added to the cell and then there is a constant supply of oxygen. Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is.. How Do Hydrogen Fuel Cells Work Gcse.
From www.chfca.ca
About Fuel Cells CHFCA How Do Hydrogen Fuel Cells Work Gcse A chemical cell produces a voltage until one of the reactants is used up. Fuel cells work by using hydrogen and oxygen to produce an electrical current. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. The hydrogen is fed into the anode (negative electrode) of the fuel. How Do Hydrogen Fuel Cells Work Gcse.
From www.air101.co.uk
Air101 Hydrogen fuel cells, explained How Do Hydrogen Fuel Cells Work Gcse Fuel cells work by using hydrogen and oxygen to produce an electrical current. A chemical cell produces a voltage until one of the reactants is used up. The fuel is added to the cell and then there is a constant supply of oxygen. The fuel is oxidised electrochemically within the fuel cell. How does a hydrogen fuel cell work? A. How Do Hydrogen Fuel Cells Work Gcse.
From butane.chem.uiuc.edu
Hydrogen Fuel Cell, High School How Do Hydrogen Fuel Cells Work Gcse A chemical cell produces a voltage until one of the reactants is used up. Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. Fuel cells work by using hydrogen and oxygen to produce an electrical current. The fuel is added to the cell and then there is a constant supply of oxygen. Revision notes on 5.4.2. How Do Hydrogen Fuel Cells Work Gcse.
From www.hydrogenfuelnews.com
How Does Hydrogen Fuel Cells Work Hydrogen Fuel News How Do Hydrogen Fuel Cells Work Gcse Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. The hydrogen is fed into the anode (negative electrode) of. How Do Hydrogen Fuel Cells Work Gcse.
From www.hydrogenfuelnews.com
How Does Hydrogen Fuel Cells Work Hydrogen Fuel News How Do Hydrogen Fuel Cells Work Gcse Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. →how does a fuel cell work? A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is an electrochemical. How Do Hydrogen Fuel Cells Work Gcse.
From energytheory.com
Hydrogen Fuel Cells Explained Efficiency and Working Energy Theory How Do Hydrogen Fuel Cells Work Gcse Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. The fuel is added to the cell and then there is a constant supply of oxygen. Fuel cells work by using hydrogen and oxygen to produce an electrical current. →how does a fuel cell work? A fuel cell is an electrochemical cell in which a fuel donates. How Do Hydrogen Fuel Cells Work Gcse.
From www.tffn.net
Exploring How Does a Hydrogen Fuel Cell Work A Comprehensive Guide How Do Hydrogen Fuel Cells Work Gcse A chemical cell produces a voltage until one of the reactants is used up. →how does a fuel cell work? A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. The fuel is oxidised electrochemically within the fuel cell. The fuel is added to the cell and then there is a. How Do Hydrogen Fuel Cells Work Gcse.
From www.alamy.com
How fuel cells work to produce electricity from hydrogen and air Stock How Do Hydrogen Fuel Cells Work Gcse The fuel is oxidised electrochemically within the fuel cell. The fuel is added to the cell and then there is a constant supply of oxygen. A chemical cell produces a voltage until one of the reactants is used up. The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. A fuel cell is. How Do Hydrogen Fuel Cells Work Gcse.
From www.cummins.com
Five key questions about the next frontier Hydrogen fuel cells How Do Hydrogen Fuel Cells Work Gcse Fuel cells work by using hydrogen and oxygen to produce an electrical current. →how does a fuel cell work? How does a hydrogen fuel cell work? The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. A chemical cell produces a voltage until one of the reactants is used up. Supplied by an. How Do Hydrogen Fuel Cells Work Gcse.
From www.researchgate.net
Schematic diagram of hydrogen fuel cell Download Scientific Diagram How Do Hydrogen Fuel Cells Work Gcse Fuel cells work by using hydrogen and oxygen to produce an electrical current. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The fuel is added to the cell and then there is a constant supply of oxygen. The fuel is oxidised electrochemically within the fuel cell. How does a hydrogen fuel. How Do Hydrogen Fuel Cells Work Gcse.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica How Do Hydrogen Fuel Cells Work Gcse Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. How does a hydrogen fuel cell work? A chemical cell produces a voltage until one of the reactants is used up. The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. Revision notes on 5.4.2 fuel. How Do Hydrogen Fuel Cells Work Gcse.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working How Do Hydrogen Fuel Cells Work Gcse Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. A chemical cell produces a voltage until one of the reactants is used up. A chemical cell produces a voltage until one of the reactants is used up. Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. The hydrogen. How Do Hydrogen Fuel Cells Work Gcse.
From www.youtube.com
How hydrogen fuel cell works Fuel Cell Technology Working principle How Do Hydrogen Fuel Cells Work Gcse Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. A chemical cell produces a voltage until one of the reactants is used up. The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. →how does a fuel cell work? Revision notes on 5.4.2 fuel cells. How Do Hydrogen Fuel Cells Work Gcse.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars How Do Hydrogen Fuel Cells Work Gcse Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. →how does a fuel cell work? A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. A chemical cell produces a voltage until one of the reactants is used up. Fuel cells work by using hydrogen and oxygen. How Do Hydrogen Fuel Cells Work Gcse.
From www.youtube.com
How does a hydrogen fuel cell work? / ¿Cómo funciona una pila de How Do Hydrogen Fuel Cells Work Gcse The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. How does a hydrogen fuel cell work? →how does a fuel cell work? A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. The fuel is oxidised electrochemically within the fuel cell. Supplied by. How Do Hydrogen Fuel Cells Work Gcse.
From www.tffn.net
How Does Hydrogen Fuel Cells Work? A Comprehensive Guide The How Do Hydrogen Fuel Cells Work Gcse The fuel is oxidised electrochemically within the fuel cell. The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Supplied by an external source of fuel (e.g hydrogen) and oxygen or air.. How Do Hydrogen Fuel Cells Work Gcse.
From pinterest.com
How a hydrogen fuel cell works. Michigan facts Pinterest How Do Hydrogen Fuel Cells Work Gcse Supplied by an external source of fuel (e.g hydrogen) and oxygen or air. The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. A chemical cell produces a voltage until one of the reactants. How Do Hydrogen Fuel Cells Work Gcse.
From www.youtube.com
How does a hydrogen fuel cell work? (AKIO TV) YouTube How Do Hydrogen Fuel Cells Work Gcse Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Fuel cells work by using hydrogen and oxygen to produce an electrical current. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. How does a hydrogen fuel cell work?. How Do Hydrogen Fuel Cells Work Gcse.
From www.linquip.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip How Do Hydrogen Fuel Cells Work Gcse Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. Fuel cells work by using hydrogen and oxygen to produce an electrical current. A chemical cell produces a voltage until one of the reactants is used up. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry. How Do Hydrogen Fuel Cells Work Gcse.
From www.tes.com
Fuel Cells GCSE AQA Teaching Resources How Do Hydrogen Fuel Cells Work Gcse A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. The fuel is added to the cell and then there is a constant supply of oxygen. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Fuel cells work by. How Do Hydrogen Fuel Cells Work Gcse.
From www.sundyne.com
Hydrogen Fuel Cells How Do They Work? Sundyne How Do Hydrogen Fuel Cells Work Gcse A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains. →how does a fuel cell work? A chemical cell produces a voltage until one of the reactants is used up. How does a hydrogen fuel cell work? Fuel cells work by using hydrogen and oxygen to produce an electrical current. A. How Do Hydrogen Fuel Cells Work Gcse.
From www.tffn.net
How Does Hydrogen Fuel Cells Work? A Comprehensive Guide The How Do Hydrogen Fuel Cells Work Gcse A chemical cell produces a voltage until one of the reactants is used up. Fuel cells work by using hydrogen and oxygen to produce an electrical current. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Fuel cells produce electrical energy using a reaction between an external fuel. How Do Hydrogen Fuel Cells Work Gcse.
From www.slideserve.com
PPT Major Advantages of Hydrogen Fuel Cell Technology PowerPoint How Do Hydrogen Fuel Cells Work Gcse The fuel is oxidised electrochemically within the fuel cell. The fuel is added to the cell and then there is a constant supply of oxygen. The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. Fuel. How Do Hydrogen Fuel Cells Work Gcse.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube How Do Hydrogen Fuel Cells Work Gcse The hydrogen is fed into the anode (negative electrode) of the fuel cell and the oxygen is. →how does a fuel cell work? A chemical cell produces a voltage until one of the reactants is used up. A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is an electrochemical cell in which. How Do Hydrogen Fuel Cells Work Gcse.