Calorimeter Theory . The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. It uses devices called calorimeters, which measure the change in. This chapter introduces calorimetry, the measurement of the quantity of heat , and the contemporary theory of heat, the caloric theory.
from mechasource.blogspot.com
Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. By knowing the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. This chapter introduces calorimetry, the measurement of the quantity of heat , and the contemporary theory of heat, the caloric theory. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the change in. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry.
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas
Calorimeter Theory Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. This chapter introduces calorimetry, the measurement of the quantity of heat , and the contemporary theory of heat, the caloric theory. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. By knowing the change in. It uses devices called calorimeters, which measure the change in. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic.
From grade12uchemistry.weebly.com
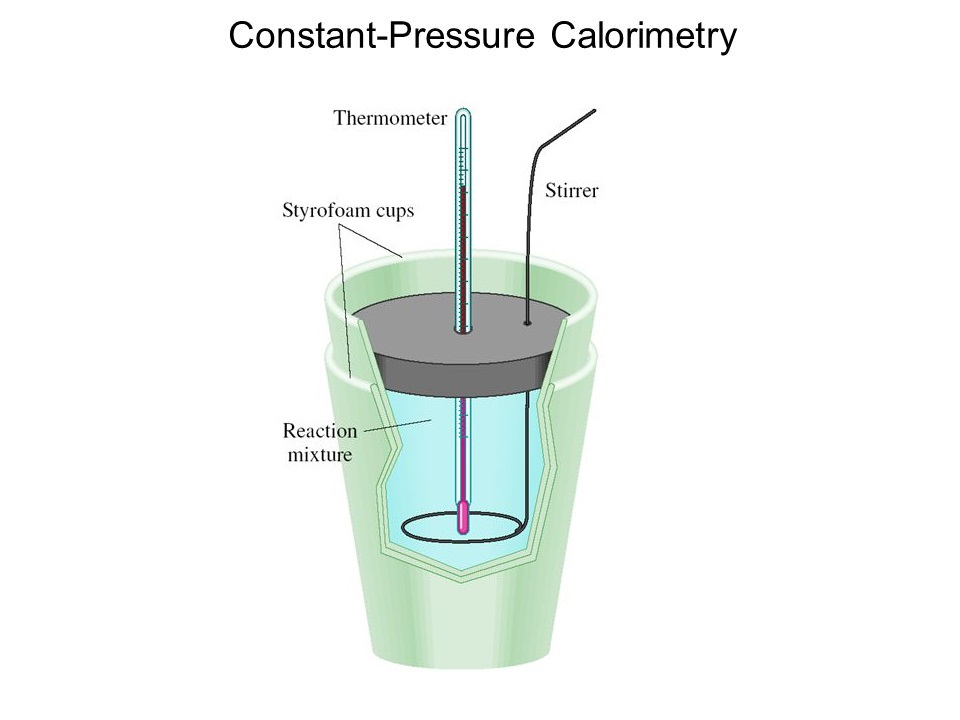
Calorimetry Grade12UChemistry Calorimeter Theory By knowing the change in. It uses devices called calorimeters, which measure the change in. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. The act or. Calorimeter Theory.
From www.youtube.com
principe fonctionnement calorimetre YouTube Calorimeter Theory Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. Calorimeter Theory.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Theory For example, when an exothermic. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the change in. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical. Calorimeter Theory.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimeter Theory Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The act or science of measuring the changes in the state variables of a body in order to calculate. Calorimeter Theory.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Calorimeter Theory This chapter introduces calorimetry, the measurement of the quantity of heat , and the contemporary theory of heat, the caloric theory. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat. Calorimeter Theory.
From www.youtube.com
050 Calorimetry YouTube Calorimeter Theory Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. This chapter introduces calorimetry, the measurement of the quantity of heat , and the contemporary theory of heat, the. Calorimeter Theory.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Calorimeter Theory Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. The act or science of measuring the changes in the state variables of a body in order to. Calorimeter Theory.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Theory The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected. Calorimeter Theory.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Theory Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. It uses devices called calorimeters, which measure the change in. This chapter introduces calorimetry, the measurement of the quantity of heat. Calorimeter Theory.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Theory It uses devices called calorimeters, which measure the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. Calorimetry is the measurement of the transfer of heat. Calorimeter Theory.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Theory Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. By. Calorimeter Theory.
From www.slideserve.com
PPT CALORIC THEORY OF HEAT PowerPoint Presentation ID1233394 Calorimeter Theory For example, when an exothermic. It uses devices called calorimeters, which measure the change in. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. The. Calorimeter Theory.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Theory This chapter introduces calorimetry, the measurement of the quantity of heat , and the contemporary theory of heat, the caloric theory. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. By knowing the change in. Calorimetry is the process of measuring the amount of heat released or. Calorimeter Theory.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Theory Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the change in. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical. Calorimeter Theory.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Calorimeter Theory This chapter introduces calorimetry, the measurement of the quantity of heat , and the contemporary theory of heat, the caloric theory. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure. Calorimeter Theory.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Theory Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. By. Calorimeter Theory.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Calorimeter Theory By knowing the change in. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. For example, when an exothermic. This chapter introduces calorimetry, the measurement of the. Calorimeter Theory.
From saylordotorg.github.io
Calorimetry Calorimeter Theory Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. By knowing the change in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The act or science. Calorimeter Theory.
From kaffee.50webs.com
Lab Calorimetry Calorimeter Theory A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. By knowing the change in. This chapter. Calorimeter Theory.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Theory For example, when an exothermic. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to. Calorimeter Theory.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Calorimeter Theory A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. By knowing the change in. Calorimetry is the measurement of the transfer of heat into or out of a system during. Calorimeter Theory.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo Calorimeter Theory It uses devices called calorimeters, which measure the change in. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. By knowing the change in. A. Calorimeter Theory.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimeter Theory For example, when an exothermic. This chapter introduces calorimetry, the measurement of the quantity of heat , and the contemporary theory of heat, the caloric theory. It uses devices called calorimeters, which measure the change in. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. The act or science. Calorimeter Theory.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter Theory Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. By knowing the change in. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. The act or science of measuring the changes in the state variables of a body in order to calculate the heat. Calorimeter Theory.
From www.researchgate.net
A schematic of the calorimeterbased method for the thermal Calorimeter Theory The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. Calorimeter Theory.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimeter Theory Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. It uses devices called calorimeters, which measure the change in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. Thermal response coefficients are thermal properties. Calorimeter Theory.
From www.jove.com
Thermochemistry Constant Volume Calorimetry JoVE Book Calorimeter Theory The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected. Calorimeter Theory.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Theory This chapter introduces calorimetry, the measurement of the quantity of heat , and the contemporary theory of heat, the caloric theory. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is. Calorimeter Theory.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Theory Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This chapter introduces calorimetry, the measurement of the quantity of heat , and the contemporary theory of heat, the. Calorimeter Theory.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimeter Theory Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. By. Calorimeter Theory.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Calorimeter Theory For example, when an exothermic. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. By knowing the change in. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as. Calorimeter Theory.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimeter Theory Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic. The act or science of measuring the changes in the state variables of a body in order to calculate. Calorimeter Theory.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Theory It uses devices called calorimeters, which measure the change in. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer associated with changes in its states, such as physical changes or phase transitions under specific conditions, is known as calorimetry. A calorimeter is a device used to measure. Calorimeter Theory.
From www.science-revision.co.uk
Calorimetry Calorimeter Theory It uses devices called calorimeters, which measure the change in. By knowing the change in. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is the process of measuring the amount of heat released. Calorimeter Theory.
From users.highland.edu
Calorimetry Calorimeter Theory This chapter introduces calorimetry, the measurement of the quantity of heat , and the contemporary theory of heat, the caloric theory. Thermal response coefficients are thermal properties of matter that characterise the response of a system that is subjected to a. It uses devices called calorimeters, which measure the change in. A calorimeter is a device used to measure the. Calorimeter Theory.