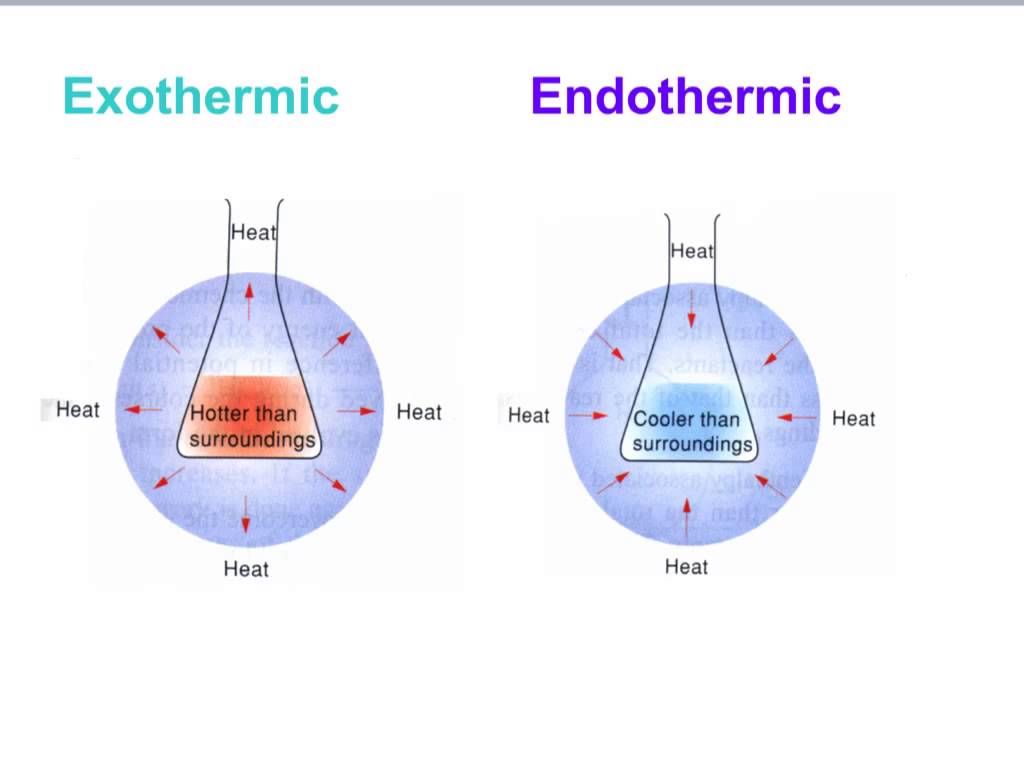
Is Water Cooling Endothermic Or Exothermic . Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. Atoms are held together by a certain amount of energy called bond energy. An endothermic process would imply that heat must be supplied to. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. Chemical processes are labeled as exothermic or endothermic. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process.
from chhattisgarh.pscnotes.com
We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. Atoms are held together by a certain amount of energy called bond energy. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. Chemical processes are labeled as exothermic or endothermic. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. An endothermic process would imply that heat must be supplied to.
Exothermic and endothermic reactions CGPCS Exam Preparation
Is Water Cooling Endothermic Or Exothermic We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. Chemical processes are labeled as exothermic or endothermic. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. Atoms are held together by a certain amount of energy called bond energy. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. An endothermic process would imply that heat must be supplied to.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Is Water Cooling Endothermic Or Exothermic We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. An endothermic process would imply that heat must be supplied to. Atoms are held together by a certain amount of. Is Water Cooling Endothermic Or Exothermic.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Is Water Cooling Endothermic Or Exothermic Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. An endothermic process would imply that heat must be supplied to. Atoms are held together by a certain amount of energy called bond. Is Water Cooling Endothermic Or Exothermic.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube Is Water Cooling Endothermic Or Exothermic The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. An endothermic process would imply. Is Water Cooling Endothermic Or Exothermic.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Is Water Cooling Endothermic Or Exothermic Chemical processes are labeled as exothermic or endothermic. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. An endothermic process would imply that heat must be supplied to. The reaction system to be considered is only the water, and if water is cooling down, it must be. Is Water Cooling Endothermic Or Exothermic.
From www.thoughtco.com
Endothermic Reaction Examples Is Water Cooling Endothermic Or Exothermic Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. Chemical processes are labeled as. Is Water Cooling Endothermic Or Exothermic.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Is Water Cooling Endothermic Or Exothermic An endothermic process would imply that heat must be supplied to. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. Atoms are held together by a certain amount of energy called bond energy. As aresult,. Is Water Cooling Endothermic Or Exothermic.
From chhattisgarh.pscnotes.com
Exothermic and endothermic reactions CGPCS Exam Preparation Is Water Cooling Endothermic Or Exothermic Chemical processes are labeled as exothermic or endothermic. An endothermic process would imply that heat must be supplied to. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. Atoms are held together by a certain amount of energy called bond energy. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when. Is Water Cooling Endothermic Or Exothermic.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Is Water Cooling Endothermic Or Exothermic Atoms are held together by a certain amount of energy called bond energy. An endothermic process would imply that heat must be supplied to. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. The reaction system to be considered. Is Water Cooling Endothermic Or Exothermic.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4499939 Is Water Cooling Endothermic Or Exothermic Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. Atoms are held together by a certain amount of energy called bond energy. An endothermic process would imply that heat must be supplied. Is Water Cooling Endothermic Or Exothermic.
From sciencenotes.org
Exothermic Reactions Definition and Examples Is Water Cooling Endothermic Or Exothermic We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. Atoms are held together by a certain amount of energy called bond energy. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. The reaction system to be considered is. Is Water Cooling Endothermic Or Exothermic.
From ar.inspiredpencil.com
Endothermic And Exothermic Phase Changes Is Water Cooling Endothermic Or Exothermic As aresult, the frezing of water is an exothermic process because heat is being removed from the system. Chemical processes are labeled as exothermic or endothermic. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. Atoms are held together by a certain amount of energy called bond energy. We will formally introduce endothermic and exothermic reactions in. Is Water Cooling Endothermic Or Exothermic.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Is Water Cooling Endothermic Or Exothermic Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. Atoms are held together by a certain amount of energy called bond energy. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. Chemical processes are labeled as exothermic or endothermic. The reaction system to be. Is Water Cooling Endothermic Or Exothermic.
From a-z-animals.com
Endothermic vs. Exothermic Reactions Key Differences and Examples A Is Water Cooling Endothermic Or Exothermic Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. Chemical. Is Water Cooling Endothermic Or Exothermic.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Is Water Cooling Endothermic Or Exothermic The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. Atoms. Is Water Cooling Endothermic Or Exothermic.
From eduinput.com
Difference Between Endothermic And Exothermic Reactions Is Water Cooling Endothermic Or Exothermic Atoms are held together by a certain amount of energy called bond energy. Chemical processes are labeled as exothermic or endothermic. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. An endothermic process would imply that heat must be. Is Water Cooling Endothermic Or Exothermic.
From mavink.com
Endothermic And Exothermic Reactions Is Water Cooling Endothermic Or Exothermic Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. Chemical processes are labeled as exothermic or endothermic. Atoms are held together by a certain amount of energy called bond energy. An endothermic process would imply that heat must be. Is Water Cooling Endothermic Or Exothermic.
From opentextbc.ca
Phase Changes Basic HVAC Is Water Cooling Endothermic Or Exothermic We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. Atoms are held together by a certain amount of energy called bond energy. An endothermic process would imply that heat. Is Water Cooling Endothermic Or Exothermic.
From facts.net
16 Intriguing Facts About Endothermic Is Water Cooling Endothermic Or Exothermic Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. An. Is Water Cooling Endothermic Or Exothermic.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Is Water Cooling Endothermic Or Exothermic We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. The reaction system to be considered is only. Is Water Cooling Endothermic Or Exothermic.
From studymind.co.uk
Endothermic vs Exothermic Reactions (GCSE Chemistry) Study Mind Is Water Cooling Endothermic Or Exothermic As aresult, the frezing of water is an exothermic process because heat is being removed from the system. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing. Is Water Cooling Endothermic Or Exothermic.
From www.youtube.com
Exothermic and Endothermic Reactions and the Math Associated with Them Is Water Cooling Endothermic Or Exothermic We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. Chemical. Is Water Cooling Endothermic Or Exothermic.
From gy.kimiq.com
In An Exothermic Reaction Energy Is Transferred From Energy Etfs Is Water Cooling Endothermic Or Exothermic Chemical processes are labeled as exothermic or endothermic. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. An endothermic process would imply that heat must be supplied to. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when. Is Water Cooling Endothermic Or Exothermic.
From letstalkscience.ca
The Cold Pack A Chilly Example of an Endothermic Reaction Let's Talk Is Water Cooling Endothermic Or Exothermic We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. Atoms are held together by a certain amount of energy called bond energy.. Is Water Cooling Endothermic Or Exothermic.
From www.pinterest.co.uk
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Is Water Cooling Endothermic Or Exothermic Chemical processes are labeled as exothermic or endothermic. Atoms are held together by a certain amount of energy called bond energy. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. An endothermic process would imply that heat must be. Is Water Cooling Endothermic Or Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Is Water Cooling Endothermic Or Exothermic Atoms are held together by a certain amount of energy called bond energy. Chemical processes are labeled as exothermic or endothermic. An endothermic process would imply that heat must be supplied to. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when. Is Water Cooling Endothermic Or Exothermic.
From higheducationlearning.com
Is Sublimation Endothermic Or Exothermic Process? » Education Tips Is Water Cooling Endothermic Or Exothermic We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. An endothermic process would imply that heat must be supplied to. Atoms are held together by a certain amount of energy called bond energy. As aresult, the frezing of water is an exothermic process because heat is being. Is Water Cooling Endothermic Or Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Is Water Cooling Endothermic Or Exothermic Atoms are held together by a certain amount of energy called bond energy. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. As aresult, the frezing of water is an exothermic process because heat is. Is Water Cooling Endothermic Or Exothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Is Water Cooling Endothermic Or Exothermic We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. Atoms. Is Water Cooling Endothermic Or Exothermic.
From stock.adobe.com
Endothermic Vs Exothermic Reactions Infographic Diagram showing a Is Water Cooling Endothermic Or Exothermic Atoms are held together by a certain amount of energy called bond energy. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. Chemical processes are labeled as exothermic or endothermic. An endothermic process would imply that heat must be supplied to. Exothermic processes before diving into freezing, let’s define endothermic and. Is Water Cooling Endothermic Or Exothermic.
From 7esl.com
Exothermic vs. Endothermic The Main Difference • 7ESL Is Water Cooling Endothermic Or Exothermic Atoms are held together by a certain amount of energy called bond energy. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we discuss the first law of. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process.. Is Water Cooling Endothermic Or Exothermic.
From mungfali.com
Exothermic Vs Endothermic Diagram Is Water Cooling Endothermic Or Exothermic Chemical processes are labeled as exothermic or endothermic. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. An endothermic process would imply that heat must be supplied to. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. We will formally introduce endothermic and exothermic reactions in the section. Is Water Cooling Endothermic Or Exothermic.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic Is Water Cooling Endothermic Or Exothermic Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. An endothermic process would imply that heat must be supplied to. As aresult, the frezing of water is an exothermic process because heat. Is Water Cooling Endothermic Or Exothermic.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Is Water Cooling Endothermic Or Exothermic Atoms are held together by a certain amount of energy called bond energy. Chemical processes are labeled as exothermic or endothermic. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. An endothermic process would imply that heat must be supplied to. The reaction system to be considered is only the water,. Is Water Cooling Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Is Water Cooling Endothermic Or Exothermic Chemical processes are labeled as exothermic or endothermic. Atoms are held together by a certain amount of energy called bond energy. The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. Exothermic processes before diving into freezing, let’s define endothermic and exothermic processes. As aresult,. Is Water Cooling Endothermic Or Exothermic.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Is Water Cooling Endothermic Or Exothermic The reaction system to be considered is only the water, and if water is cooling down, it must be releasing energy in an exothermic process. As aresult, the frezing of water is an exothermic process because heat is being removed from the system. We will formally introduce endothermic and exothermic reactions in the section 5.4 (the next section) when we. Is Water Cooling Endothermic Or Exothermic.