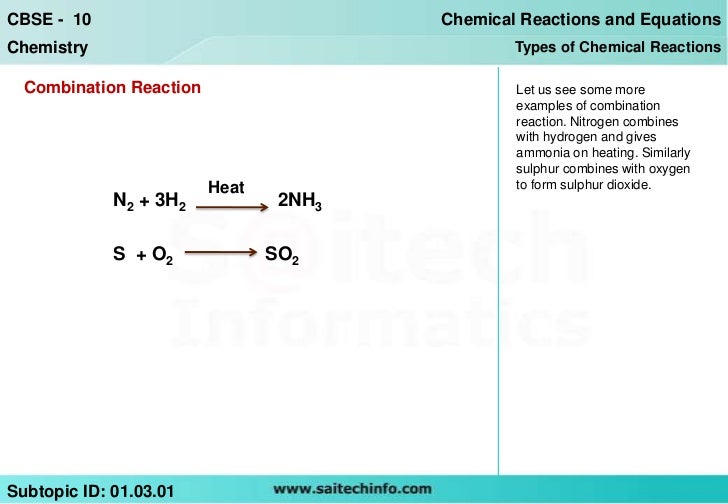
Combination Reaction Formula . A combination reaction is a reaction in which two or more substances combine to form a single new. In this form, a synthesis reaction is easy to recognize because you have more reactants than products. \[a + b \rightarrow c\] in which two or more reactants become one product (are. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. A + b → ab. In a synthesis reaction, two or more chemical species combine to form a more complex product: A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. Two or more reactants combine to make one larger compound. Combination reactions describe a reaction like this: In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product.
from www.slideshare.net
In this form, a synthesis reaction is easy to recognize because you have more reactants than products. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. A combination reaction is a reaction in which two or more substances combine to form a single new. A + b → ab. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. \[a + b \rightarrow c\] in which two or more reactants become one product (are. Combination reactions describe a reaction like this: In a synthesis reaction, two or more chemical species combine to form a more complex product: In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. Two or more reactants combine to make one larger compound.
Combination reaction
Combination Reaction Formula A + b → ab. A + b → ab. In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. A combination reaction is a reaction in which two or more substances combine to form a single new. \[a + b \rightarrow c\] in which two or more reactants become one product (are. Two or more reactants combine to make one larger compound. Combination reactions describe a reaction like this: In this form, a synthesis reaction is easy to recognize because you have more reactants than products. In a synthesis reaction, two or more chemical species combine to form a more complex product:
From sciencenotes.org
What Is a Synthesis Reaction? Definition and Examples Combination Reaction Formula In a synthesis reaction, two or more chemical species combine to form a more complex product: \[a + b \rightarrow c\] in which two or more reactants become one product (are. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. A combination reaction is a reaction in which two or. Combination Reaction Formula.
From byjus.com
Types of Chemical Reactions Detailed Explanation With Example & Videos Combination Reaction Formula Two or more reactants combine to make one larger compound. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. In this form,. Combination Reaction Formula.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Combination Reaction Formula A combination reaction is a reaction in which two or more substances combine to form a single new. \[a + b \rightarrow c\] in which two or more reactants become one product (are. In a synthesis reaction, two or more chemical species combine to form a more complex product: Learn how to write and balance chemical equations for combination reactions,. Combination Reaction Formula.
From www.slideserve.com
PPT BALANCING EQUATIONS & CHEMICAL REACTIONS PowerPoint Presentation Combination Reaction Formula In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. Two or more reactants combine to make one larger compound. A + b → ab. A combination reaction is a reaction in which two or more substances combine to form a single. Combination Reaction Formula.
From www.youtube.com
what is Combination reaction? class 10 chemical reaction and Combination Reaction Formula A combination reaction is a reaction in which two or more substances combine to form a single new. A + b → ab. Combination reactions describe a reaction like this: Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. In a synthesis reaction, two or more chemical species combine to. Combination Reaction Formula.
From www.youtube.com
Chemical Reaction and Equation Reaction) class 10th Combination Reaction Formula In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. In a synthesis reaction, two or more chemical species combine to form a. Combination Reaction Formula.
From www.youtube.com
Combination Reaction Class 10 Chemistry Chemical Reactions and Combination Reaction Formula A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. A + b → ab. Two or more reactants combine to make one. Combination Reaction Formula.
From www.youtube.com
Combination Reactions YouTube Combination Reaction Formula Two or more reactants combine to make one larger compound. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. A + b → ab. In a synthesis reaction, two or more. Combination Reaction Formula.
From buchanonlibrary.blogspot.com
Combination Reaction Worksheet 1 Buchanon Library Combination Reaction Formula \[a + b \rightarrow c\] in which two or more reactants become one product (are. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is. Combination Reaction Formula.
From geteducationskills.com
Latest Chapter on Combination Reaction With Example Get Education Combination Reaction Formula A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. A + b → ab. Two or more reactants combine to make one larger compound. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. In this form, a synthesis reaction is. Combination Reaction Formula.
From www.slideshare.net
Combination reaction Combination Reaction Formula In a synthesis reaction, two or more chemical species combine to form a more complex product: In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two. Combination Reaction Formula.
From www.teachoo.com
NCERT Exemplar (MCQ) Which of the following are combination reaction Combination Reaction Formula A combination reaction is a reaction in which two or more substances combine to form a single new. In a synthesis reaction, two or more chemical species combine to form a more complex product: In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is. Combination Reaction Formula.
From study.com
How to Identify a Combination Reaction Chemistry Combination Reaction Formula \[a + b \rightarrow c\] in which two or more reactants become one product (are. In this form, a synthesis reaction is easy to recognize because you have more reactants than products. In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product.. Combination Reaction Formula.
From www.youtube.com
Writing equations for reactions YouTube Combination Reaction Formula In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. In a synthesis reaction, two or more chemical species combine to form a more complex product: Two or more reactants combine to make one larger compound. A combination reaction is a reaction. Combination Reaction Formula.
From www.teachoo.com
Combination Reaction Definition with 4 Examples Class 10 Chemistry Combination Reaction Formula Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. In this form, a synthesis reaction is easy to recognize because you have. Combination Reaction Formula.
From www.slideserve.com
PPT 7.57.6 Types of Reactions and Combination Reactions PowerPoint Combination Reaction Formula \[a + b \rightarrow c\] in which two or more reactants become one product (are. A + b → ab. In a synthesis reaction, two or more chemical species combine to form a more complex product: In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and. Combination Reaction Formula.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation ID909983 Combination Reaction Formula Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. A combination reaction is a reaction in which two or more substances combine to form a single new. In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants. Combination Reaction Formula.
From www.slideserve.com
PPT Rules for Balancing Equations PowerPoint Presentation, free Combination Reaction Formula A combination reaction is a reaction in which two or more substances combine to form a single new. Combination reactions describe a reaction like this: \[a + b \rightarrow c\] in which two or more reactants become one product (are. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. In. Combination Reaction Formula.
From www.chemistrylearner.com
Chemical Reactions Types, Definitions, and Examples Combination Reaction Formula \[a + b \rightarrow c\] in which two or more reactants become one product (are. Combination reactions describe a reaction like this: In this form, a synthesis reaction is easy to recognize because you have more reactants than products. Two or more reactants combine to make one larger compound. Learn how to write and balance chemical equations for combination reactions,. Combination Reaction Formula.
From www.filscihub.com
[CHEMISTRY MODULE] Combination Reactions — Filipino Science Hub Combination Reaction Formula A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. A combination reaction is a reaction in which two or more substances combine to form a single new. A + b →. Combination Reaction Formula.
From studyqueries.com
What Is Combination Reaction? Definition, Examples Combination Reaction Formula A + b → ab. In this form, a synthesis reaction is easy to recognize because you have more reactants than products. A combination reaction is a reaction in which two or more substances combine to form a single new. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. Combination. Combination Reaction Formula.
From www.slideserve.com
PPT Chapter 8 Chemical Reactions PowerPoint Presentation, free Combination Reaction Formula Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. A + b → ab. A combination reaction is a reaction in which. Combination Reaction Formula.
From www.vrogue.co
What Is Meant By Addition Reaction Give An Example Wi vrogue.co Combination Reaction Formula A + b → ab. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. Combination reactions describe a reaction like this: In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. In. Combination Reaction Formula.
From www.slideserve.com
PPT Classifying Chemical Reactions PowerPoint Presentation, free Combination Reaction Formula A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. In this form, a synthesis reaction is easy to recognize because you have more reactants than products. A + b → ab. In a combination reaction, the general formula can be represented as a + b → ab, where a and. Combination Reaction Formula.
From colouremployer8.gitlab.io
Supreme 4 Types Of Chemical Reactions Reactants And Products Cellular Combination Reaction Formula A + b → ab. In a synthesis reaction, two or more chemical species combine to form a more complex product: In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. Learn how to write and balance chemical equations for combination reactions,. Combination Reaction Formula.
From www.slideserve.com
PPT Unit 7 Chemical Equations PowerPoint Presentation, free download Combination Reaction Formula Two or more reactants combine to make one larger compound. A combination reaction is a reaction in which two or more substances combine to form a single new. \[a + b \rightarrow c\] in which two or more reactants become one product (are. A + b → ab. Combination reactions describe a reaction like this: In this form, a synthesis. Combination Reaction Formula.
From www.youtube.com
Types of Chemical Equations with Examples Combination Reaction Formula A + b → ab. Two or more reactants combine to make one larger compound. In a synthesis reaction, two or more chemical species combine to form a more complex product: A combination reaction is a reaction in which two or more substances combine to form a single new. \[a + b \rightarrow c\] in which two or more reactants. Combination Reaction Formula.
From drcalef.com
Combination (Synthesis) Reactions Combination Reaction Formula In this form, a synthesis reaction is easy to recognize because you have more reactants than products. A combination reaction is a reaction in which two or more substances combine to form a single new. A + b → ab. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. In. Combination Reaction Formula.
From www.youtube.com
Chemical reactions and Equation Combination Reactions YouTube Combination Reaction Formula A + b → ab. A combination reaction is a reaction in which two or more substances combine to form a single new. Two or more reactants combine to make one larger compound. \[a + b \rightarrow c\] in which two or more reactants become one product (are. Combination reactions describe a reaction like this: In a combination reaction, the. Combination Reaction Formula.
From www.youtube.com
Combination reaction Chemical reactions and equations CLASS 10 Ch Combination Reaction Formula Combination reactions describe a reaction like this: In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the reactants and ab is the product. \[a + b \rightarrow c\] in which two or more reactants become one product (are. A + b → ab. A combination reaction is a. Combination Reaction Formula.
From www.thoughtco.com
Types of Chemical Reactions (With Examples) Combination Reaction Formula In a synthesis reaction, two or more chemical species combine to form a more complex product: Two or more reactants combine to make one larger compound. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. \[a + b \rightarrow c\] in which two or more reactants become one product (are.. Combination Reaction Formula.
From slidetodoc.com
Chemical Equations Reactions Chemistry A Chemical Reactions You Combination Reaction Formula A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. In a combination reaction, the general formula can be represented as a + b → ab, where a and b are the. Combination Reaction Formula.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Combination Reaction Formula A combination reaction is a reaction in which two or more substances combine to form a single new. In this form, a synthesis reaction is easy to recognize because you have more reactants than products. Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. A + b → ab. Two. Combination Reaction Formula.
From www.slideserve.com
PPT Ch. 9 Chemical Reactions & Equations PowerPoint Presentation ID Combination Reaction Formula Learn how to write and balance chemical equations for combination reactions, where two reactants combine to form one product. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple. In a synthesis reaction, two or more chemical species combine to form a more complex product: In this form, a synthesis reaction. Combination Reaction Formula.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Combination Reaction Formula Two or more reactants combine to make one larger compound. A combination reaction is a reaction in which two or more substances combine to form a single new. In a synthesis reaction, two or more chemical species combine to form a more complex product: A + b → ab. A synthesis reaction or direct combination reaction is a type of. Combination Reaction Formula.