Medical Devices Testing Procedures . This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Initially, the concept is scrutinized for viability, safety, and market need. Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. The process for medical device testing is thorough and methodical. Explore admet's comprehensive solutions for the mechanical testing of medical devices. Scheduled quality assurance testing is. Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. How do you want a medical device to work if you or someone you love is the next patient to be connected to it? Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Medical device testing and qa. Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards.
from www.orielstat.com
Initially, the concept is scrutinized for viability, safety, and market need. Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. The process for medical device testing is thorough and methodical. Scheduled quality assurance testing is. Medical device testing and qa. Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. Explore admet's comprehensive solutions for the mechanical testing of medical devices.
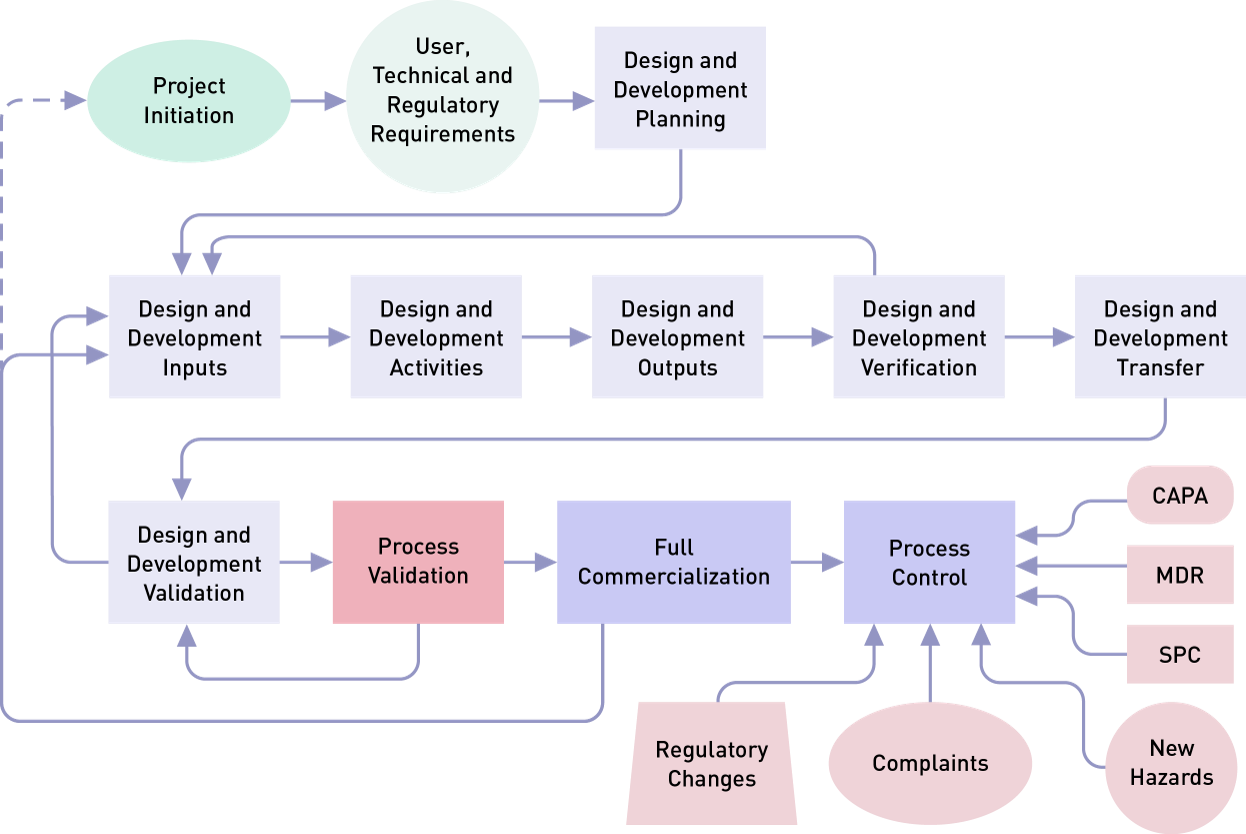
Medical Device Process Validation What You Need to Know
Medical Devices Testing Procedures Explore admet's comprehensive solutions for the mechanical testing of medical devices. How do you want a medical device to work if you or someone you love is the next patient to be connected to it? Explore admet's comprehensive solutions for the mechanical testing of medical devices. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. Scheduled quality assurance testing is. Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. Medical device testing and qa. The process for medical device testing is thorough and methodical. Initially, the concept is scrutinized for viability, safety, and market need.
From www.pannam.com
Ultimate Guide to Medical Device Design and Development Pannam Medical Devices Testing Procedures Explore admet's comprehensive solutions for the mechanical testing of medical devices. Scheduled quality assurance testing is. Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Ensure the quality, durability,. Medical Devices Testing Procedures.
From old.sermitsiaq.ag
Medical Device Verification And Validation Plan Template Medical Devices Testing Procedures Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. The process for medical device testing is thorough and methodical. Initially, the concept is scrutinized for viability, safety, and market need. Scheduled quality assurance. Medical Devices Testing Procedures.
From www.youtube.com
TÜV SÜD Testing for Medical Devices YouTube Medical Devices Testing Procedures Medical device testing and qa. Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. Explore admet's comprehensive solutions for the mechanical testing of medical devices. How do you want a medical device to work if you or someone you love is the next patient to be connected to it? Medical device reporting. Medical Devices Testing Procedures.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Devices Testing Procedures Initially, the concept is scrutinized for viability, safety, and market need. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. How do you want a medical device to work if you or someone you love is the next patient to be connected to it? Medical device testing refers to the. Medical Devices Testing Procedures.
From www.emcbayswater.com.au
» Medical Devices EMC and Immunity Testing EMC Bayswater Medical Devices Testing Procedures Initially, the concept is scrutinized for viability, safety, and market need. Scheduled quality assurance testing is. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Explore admet's comprehensive solutions for the mechanical testing of medical devices. The process for medical device testing is thorough and methodical. Medical device test procedures. Medical Devices Testing Procedures.
From aica.com
What Are the Different Types of Diagnostic Imaging Tests Available Medical Devices Testing Procedures This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Initially, the concept is scrutinized for viability, safety, and market need. Medical device testing and qa. The process for medical device testing is thorough and methodical. Medical device reporting (mdr) is one of the postmarket surveillance tools. Medical Devices Testing Procedures.
From www.youtube.com
Electrical Safety Testing For Medical Devices YouTube Medical Devices Testing Procedures Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Medical device testing and qa. How do you want a medical device to work if you or someone you love is the next patient to be connected to it? Explore admet's comprehensive solutions for the mechanical testing of medical devices. Medical. Medical Devices Testing Procedures.
From diagramresearch.com
The 3 Stages For Medical Device Clinical Investigation Explained Medical Devices Testing Procedures Explore admet's comprehensive solutions for the mechanical testing of medical devices. Medical device testing and qa. This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. Medical device reporting (mdr). Medical Devices Testing Procedures.
From www.pacificbiolabs.com
Bioburden Testing and Sterility Testing of Medical Devices Medical Devices Testing Procedures This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Scheduled quality assurance testing is. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. How do you want a medical device to work if you or someone. Medical Devices Testing Procedures.
From www.ul.com
Medical Laboratory Equipment Testing to the IEC 61010 Standard UL Medical Devices Testing Procedures Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. How do you want a medical device to work if you or someone you love is the next patient to be connected to it? Scheduled quality assurance testing is. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses. Medical Devices Testing Procedures.
From measurlabs.com
Medical Device Testing & Analysis Services Measurlabs Medical Devices Testing Procedures Initially, the concept is scrutinized for viability, safety, and market need. Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Explore admet's comprehensive solutions. Medical Devices Testing Procedures.
From www.qlaboratories.com
Medical Device Testing Lab Services By Q Laboratories Medical Devices Testing Procedures Initially, the concept is scrutinized for viability, safety, and market need. Explore admet's comprehensive solutions for the mechanical testing of medical devices. Medical device testing and qa. Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. Scheduled quality assurance testing is. This article will go over the process of writing test plans. Medical Devices Testing Procedures.
From www.eurofins.com.au
Sterility Testing for sterile pharmaceutical products and medical Medical Devices Testing Procedures Initially, the concept is scrutinized for viability, safety, and market need. Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. Scheduled quality assurance testing is. Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. Explore admet's comprehensive solutions for. Medical Devices Testing Procedures.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Devices Testing Procedures Explore admet's comprehensive solutions for the mechanical testing of medical devices. Scheduled quality assurance testing is. Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Medical device testing. Medical Devices Testing Procedures.
From ar.inspiredpencil.com
Medical Device Testing Medical Devices Testing Procedures Explore admet's comprehensive solutions for the mechanical testing of medical devices. Initially, the concept is scrutinized for viability, safety, and market need. Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical. Medical Devices Testing Procedures.
From www.ul.com
Custom Medical Device Testing UL Medical Devices Testing Procedures Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. Initially, the concept is scrutinized for viability, safety, and market need. Scheduled quality assurance testing is. The process for medical device. Medical Devices Testing Procedures.
From www.joharidigital.com
Medical Device Testing Types, Procedures, and Best Practices Medical Devices Testing Procedures Explore admet's comprehensive solutions for the mechanical testing of medical devices. How do you want a medical device to work if you or someone you love is the next patient to be connected to it? Initially, the concept is scrutinized for viability, safety, and market need. Medical device testing and qa. Medical device reporting (mdr) is one of the postmarket. Medical Devices Testing Procedures.
From flamlabelthema.netlify.app
Medical Device Test Method Validation Template Medical Devices Testing Procedures Scheduled quality assurance testing is. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. The process for medical device testing is thorough and methodical. Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. Medical device testing and qa. Initially, the concept is. Medical Devices Testing Procedures.
From www.presentationeze.com
Medical Device Instructions for Use Information & TrainingPresentationEZE Medical Devices Testing Procedures The process for medical device testing is thorough and methodical. Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. How do you want a medical device to work if you or someone you love is the next patient to be connected to it? Scheduled quality assurance testing is. Ensure the quality, durability,. Medical Devices Testing Procedures.
From courses.lumenlearning.com
Testing the Effectiveness of Antiseptics and Disinfectants Microbiology Medical Devices Testing Procedures Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Scheduled quality assurance testing is. Medical device testing and qa. This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Explore admet's comprehensive solutions for the mechanical testing. Medical Devices Testing Procedures.
From www.tentamus.com
Medical Device Testing Tentamus Group Medical Devices Testing Procedures Scheduled quality assurance testing is. The process for medical device testing is thorough and methodical. This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. Explore admet's comprehensive solutions for. Medical Devices Testing Procedures.
From www.endolab.org
Research & development of medical test methods, test protocols, test Medical Devices Testing Procedures Explore admet's comprehensive solutions for the mechanical testing of medical devices. Initially, the concept is scrutinized for viability, safety, and market need. Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. This article will go over the process of writing test plans and protocols for medical devices. Medical Devices Testing Procedures.
From www.traumamic.nihr.ac.uk
Medical Devices Testing and Evaluation Centre Trauma Management Medical Devices Testing Procedures How do you want a medical device to work if you or someone you love is the next patient to be connected to it? Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. Explore admet's comprehensive solutions for the mechanical testing of medical devices. Scheduled quality assurance testing is. Initially, the concept. Medical Devices Testing Procedures.
From www.youtube.com
Types of Test Method Validation Medical Device (Full Online Course with Medical Devices Testing Procedures The process for medical device testing is thorough and methodical. How do you want a medical device to work if you or someone you love is the next patient to be connected to it? Explore admet's comprehensive solutions for the mechanical testing of medical devices. Medical device testing and qa. Initially, the concept is scrutinized for viability, safety, and market. Medical Devices Testing Procedures.
From www.youtube.com
diagnostic medical devices in daily uses / diagnostic medical equipment Medical Devices Testing Procedures Medical device testing and qa. This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. Initially, the concept is scrutinized for viability, safety, and market. Medical Devices Testing Procedures.
From mungfali.com
Medical Device Development Process Flowchart Medical Devices Testing Procedures Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. Explore admet's comprehensive solutions for the mechanical testing of medical devices. Scheduled quality assurance testing is. Initially, the concept is scrutinized for viability, safety, and market need. The process for medical device testing is thorough and methodical. Medical. Medical Devices Testing Procedures.
From biotechanatomy.co.il
Medical Device Testing Effective Testing Procedures Biotech Medical Devices Testing Procedures Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. Medical device testing and qa. This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. The process for medical device testing is thorough and methodical.. Medical Devices Testing Procedures.
From f2labs.com
Medical Device Testing Lab EMC Testing for Medical Devices F2 Labs Medical Devices Testing Procedures Initially, the concept is scrutinized for viability, safety, and market need. Scheduled quality assurance testing is. The process for medical device testing is thorough and methodical. Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. This article will go over the process of writing test plans and protocols for medical devices in. Medical Devices Testing Procedures.
From www.testorigen.com
Reasons Why Medical Devices Need Proper Testing? TestOrigen Medical Devices Testing Procedures Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. Scheduled quality assurance testing is. Explore admet's comprehensive solutions for the mechanical testing of medical devices. The process for medical device testing is thorough and methodical. How do you want a medical device to work if you or someone you love is the. Medical Devices Testing Procedures.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Devices Testing Procedures This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. Explore admet's comprehensive solutions for the mechanical testing of medical devices. Ensure the quality, durability, and safety of medical implants,. Medical Devices Testing Procedures.
From www.youtube.com
Test Automation for Medical Devices YouTube Medical Devices Testing Procedures Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. How do you want a medical device to work if you or someone you love is the next patient to be connected to it? Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and. Medical Devices Testing Procedures.
From www.smithers.com
Medical Device Testing Video Smithers Medical Devices Testing Procedures Initially, the concept is scrutinized for viability, safety, and market need. Scheduled quality assurance testing is. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. Ensure the quality, durability, and safety of medical. Medical Devices Testing Procedures.
From www.tuvsud.com
Medical Device Testing Infographic TÜV SÜD Medical Devices Testing Procedures Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Explore admet's comprehensive solutions for the mechanical testing of medical devices. Ensure the quality, durability, and safety of medical implants, adhesives, and more with our testing systems compliant with astm and iso standards. Medical device testing and qa. This article will. Medical Devices Testing Procedures.
From www.novatx.com
Medical Device Testing Nova Biologicals Medical Devices Testing Procedures Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Medical device testing and qa. This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness,. Medical Devices Testing Procedures.
From labctl.in
Medical Device Testing Conformity Testing Labs Private Limited; NABL Medical Devices Testing Procedures Medical device test procedures are systematic processes designed to evaluate the safety, effectiveness, and performance of medical. Medical device testing refers to the process of evaluating the safety, effectiveness, and performance of medical devices. This article will go over the process of writing test plans and protocols for medical devices in line with the iec 60601 standard. Explore admet's comprehensive. Medical Devices Testing Procedures.