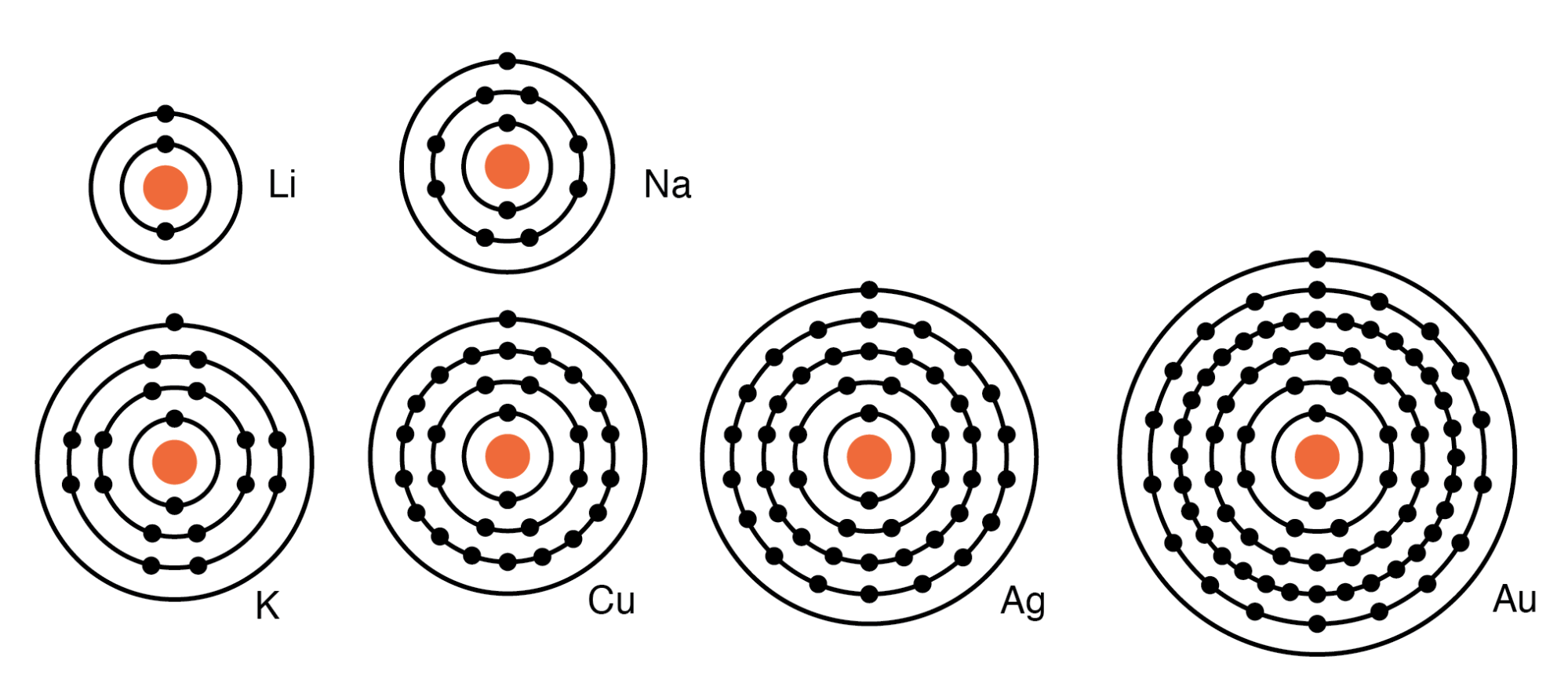
How Many Electron Shells Are In Carbon . a carbon atom has six electrons. the number of electrons in each. in an atom, the electrons spin around the center, also called the nucleus. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. The electrons like to be in separate shells/orbitals. It has two in the first shell and four in the second shell. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. learn about the periodic table, electron shells, and orbitals in this khan academy article. bohr diagrams indicate how many electrons fill each principal shell.
from www.animalia-life.club
in an atom, the electrons spin around the center, also called the nucleus. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. It has two in the first shell and four in the second shell. The electrons like to be in separate shells/orbitals. a carbon atom has six electrons. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. bohr diagrams indicate how many electrons fill each principal shell. the number of electrons in each. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. learn about the periodic table, electron shells, and orbitals in this khan academy article.
Electron Shell Diagram
How Many Electron Shells Are In Carbon learn about the periodic table, electron shells, and orbitals in this khan academy article. learn about the periodic table, electron shells, and orbitals in this khan academy article. a carbon atom has six electrons. in an atom, the electrons spin around the center, also called the nucleus. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. bohr diagrams indicate how many electrons fill each principal shell. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. The electrons like to be in separate shells/orbitals. the number of electrons in each. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. It has two in the first shell and four in the second shell.
From ar.inspiredpencil.com
Carbon Atomic Shells How Many Electron Shells Are In Carbon the number of electrons in each. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. in an atom, the electrons spin around the center, also called the nucleus. It has two in the first shell and four in the second shell. The electrons like to be in separate shells/orbitals. Group 18 elements. How Many Electron Shells Are In Carbon.
From mungfali.com
How Many Electrons Are In Each Shell How Many Electron Shells Are In Carbon in an atom, the electrons spin around the center, also called the nucleus. The electrons like to be in separate shells/orbitals. bohr diagrams indicate how many electrons fill each principal shell. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. Group 18 elements (helium, neon, and argon are shown in figure 2). How Many Electron Shells Are In Carbon.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry How Many Electron Shells Are In Carbon the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. It has two in the first shell and four in the second shell. bohr diagrams indicate how many electrons fill each principal. How Many Electron Shells Are In Carbon.
From www.alamy.com
Carbon atom hires stock photography and images Alamy How Many Electron Shells Are In Carbon a carbon atom has six electrons. in an atom, the electrons spin around the center, also called the nucleus. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. The electrons like to be in separate shells/orbitals. bohr diagrams indicate how many electrons fill each principal shell. the number of electrons. How Many Electron Shells Are In Carbon.
From www.vrogue.co
Electron Shells And Orbitals vrogue.co How Many Electron Shells Are In Carbon The electrons like to be in separate shells/orbitals. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. in an atom, the electrons spin around the center, also called the nucleus. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. . How Many Electron Shells Are In Carbon.
From www.slideserve.com
PPT Orbital Filling Electron Configurations PowerPoint Presentation, free download ID298342 How Many Electron Shells Are In Carbon learn about the periodic table, electron shells, and orbitals in this khan academy article. bohr diagrams indicate how many electrons fill each principal shell. a carbon atom has six electrons. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. for example, the electron configuration of carbon atom is written as. How Many Electron Shells Are In Carbon.
From valenceelectrons.com
Carbon(C) electron configuration and orbital diagram How Many Electron Shells Are In Carbon The electrons like to be in separate shells/orbitals. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. bohr diagrams indicate how many electrons fill each principal shell. the number of electrons in each. Group 18 elements (helium, neon, and argon are shown in figure 2). How Many Electron Shells Are In Carbon.
From ar.inspiredpencil.com
Electron Shells Carbon How Many Electron Shells Are In Carbon bohr diagrams indicate how many electrons fill each principal shell. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. a carbon atom has six electrons. It has two in the first shell and four in the second shell. the electron configurations of silicon (14. How Many Electron Shells Are In Carbon.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell How Many Electron Shells Are In Carbon Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. The electrons like to be in separate shells/orbitals. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. learn about the periodic table, electron shells, and orbitals in this khan academy article. in an atom, the electrons spin. How Many Electron Shells Are In Carbon.
From circuitwiringmoyle99.z22.web.core.windows.net
Electron Distribution Diagram Of Carbon How Many Electron Shells Are In Carbon bohr diagrams indicate how many electrons fill each principal shell. the number of electrons in each. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. It has two in the first shell and four in the second. How Many Electron Shells Are In Carbon.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer How Many Electron Shells Are In Carbon It has two in the first shell and four in the second shell. bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. in an atom, the electrons spin around the center, also called the nucleus. The electrons like to be in separate. How Many Electron Shells Are In Carbon.
From ar.inspiredpencil.com
Carbon Electron Structure How Many Electron Shells Are In Carbon It has two in the first shell and four in the second shell. The electrons like to be in separate shells/orbitals. bohr diagrams indicate how many electrons fill each principal shell. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. Group 18 elements (helium, neon, and argon are shown in figure 2) have. How Many Electron Shells Are In Carbon.
From mungfali.com
Valence Electron Shell Diagram How Many Electron Shells Are In Carbon in an atom, the electrons spin around the center, also called the nucleus. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. learn about the periodic table, electron shells, and. How Many Electron Shells Are In Carbon.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements How Many Electron Shells Are In Carbon learn about the periodic table, electron shells, and orbitals in this khan academy article. bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons. How Many Electron Shells Are In Carbon.
From www.animalia-life.club
Electron Shell Diagram How Many Electron Shells Are In Carbon the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. It has two in the first shell and. How Many Electron Shells Are In Carbon.
From chemistry291.blogspot.com
5 Steps】How Many Valence Electrons Does Carbon Have?Number of Valence Electrons in Carbon How Many Electron Shells Are In Carbon It has two in the first shell and four in the second shell. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. The electrons like to be in separate shells/orbitals. a carbon atom has six electrons. in an atom, the electrons spin around the center, also called the nucleus. learn about. How Many Electron Shells Are In Carbon.
From www.adda247.com
Valency of Carbon Check carbon valency electrons How Many Electron Shells Are In Carbon bohr diagrams indicate how many electrons fill each principal shell. the number of electrons in each. a carbon atom has six electrons. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. learn about the periodic table, electron shells, and orbitals in this khan academy article. the electron configurations of. How Many Electron Shells Are In Carbon.
From www.youtube.com
PERIODIC TABLE ELECTRON SHELLS YouTube How Many Electron Shells Are In Carbon Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. the number of electrons in each. bohr diagrams indicate how many electrons fill each principal shell. a carbon atom has six electrons. learn about the periodic table, electron shells, and orbitals in this khan academy article. for example, the electron. How Many Electron Shells Are In Carbon.
From pixels.com
Carbon Electron Configuration Photograph by Photo Libary Pixels How Many Electron Shells Are In Carbon It has two in the first shell and four in the second shell. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. The electrons like to be in separate shells/orbitals. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. learn. How Many Electron Shells Are In Carbon.
From sites.google.com
Carbon Table of Elements by Shrenil Sharma How Many Electron Shells Are In Carbon Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. bohr diagrams indicate how many electrons fill each principal shell. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. a carbon atom has six electrons. the electron configurations of. How Many Electron Shells Are In Carbon.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements How Many Electron Shells Are In Carbon a carbon atom has six electrons. in an atom, the electrons spin around the center, also called the nucleus. the number of electrons in each. learn about the periodic table, electron shells, and orbitals in this khan academy article. It has two in the first shell and four in the second shell. bohr diagrams indicate. How Many Electron Shells Are In Carbon.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology How Many Electron Shells Are In Carbon The electrons like to be in separate shells/orbitals. learn about the periodic table, electron shells, and orbitals in this khan academy article. It has two in the first shell and four in the second shell. in an atom, the electrons spin around the center, also called the nucleus. bohr diagrams indicate how many electrons fill each principal. How Many Electron Shells Are In Carbon.
From mavink.com
Carbon Electron Shell Diagram How Many Electron Shells Are In Carbon It has two in the first shell and four in the second shell. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. The electrons like to be in separate shells/orbitals. the number of electrons in each. the electron configurations of silicon (14 electrons), phosphorus (15. How Many Electron Shells Are In Carbon.
From spmkimia.onlinetuition.com.my
Susunan Elektron di Dalam Atom Nota Ulangkaji Kimia SPM Tingkatan 4/Tingkatan 5 How Many Electron Shells Are In Carbon the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. learn about the periodic table, electron shells, and orbitals in this khan academy article. the number of electrons in each. for example, the electron configuration of carbon. How Many Electron Shells Are In Carbon.
From chemistry291.blogspot.com
What Is the Carbon(C) Electron Configuration? How Many Electron Shells Are In Carbon the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. learn about the periodic table, electron shells, and orbitals in this khan academy article. bohr diagrams indicate how many electrons fill each principal shell. a carbon atom has six electrons. for example, the electron configuration of carbon atom is written as. How Many Electron Shells Are In Carbon.
From scientifictutor.org
Chem Bohr Model and Electron Shells Part 1 Scientific Tutor How Many Electron Shells Are In Carbon for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. in an atom, the electrons spin around the center, also called the nucleus. The electrons like to be in separate shells/orbitals. the number of electrons in each. learn about the periodic table, electron shells, and. How Many Electron Shells Are In Carbon.
From circuitlistzooliths99.z22.web.core.windows.net
Electron Shell Diagram For Carbon How Many Electron Shells Are In Carbon for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. bohr diagrams indicate how many electrons fill each principal shell. learn about the periodic table, electron shells, and orbitals in this khan academy article. the number of electrons in each. Group 18 elements (helium, neon,. How Many Electron Shells Are In Carbon.
From mavink.com
Carbon Electron Shell Diagram How Many Electron Shells Are In Carbon learn about the periodic table, electron shells, and orbitals in this khan academy article. in an atom, the electrons spin around the center, also called the nucleus. The electrons like to be in separate shells/orbitals. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. It. How Many Electron Shells Are In Carbon.
From mavink.com
Carbon Electron Shell Diagram How Many Electron Shells Are In Carbon Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. bohr diagrams indicate how many electrons fill each principal shell. the number of electrons in each. learn about the periodic table, electron shells, and orbitals in this khan academy article. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur. How Many Electron Shells Are In Carbon.
From ar.inspiredpencil.com
Carbon Atomic Shells How Many Electron Shells Are In Carbon Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. the number of electrons in each. It has two in the first shell and four in the second shell. bohr diagrams. How Many Electron Shells Are In Carbon.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png How Many Electron Shells Are In Carbon Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. bohr diagrams indicate how many electrons fill each principal shell. The electrons like to be in separate shells/orbitals. the number of. How Many Electron Shells Are In Carbon.
From stock.adobe.com
Molecular structure of a carbon atom. Electrons, protons, and neutrons are labeled. Nucleus and How Many Electron Shells Are In Carbon the number of electrons in each. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. The electrons like to be in separate shells/orbitals. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. in an atom, the electrons spin around the center, also called the nucleus. . How Many Electron Shells Are In Carbon.
From stock.adobe.com
Atomic elements showing the nucleus and shells, numbers of electrons, protons, and neutrons How Many Electron Shells Are In Carbon the number of electrons in each. It has two in the first shell and four in the second shell. in an atom, the electrons spin around the center, also called the nucleus. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. learn about the periodic table, electron shells, and orbitals in. How Many Electron Shells Are In Carbon.
From slideplayer.com
Electronic structure and the periodic table ppt download How Many Electron Shells Are In Carbon the number of electrons in each. It has two in the first shell and four in the second shell. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. bohr diagrams. How Many Electron Shells Are In Carbon.
From www.animalia-life.club
Electron Shell Diagram How Many Electron Shells Are In Carbon the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. for example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the k. It has two in the first shell and. How Many Electron Shells Are In Carbon.