What Temperature Does Liquid Evaporate . temperature, of course, affects how quickly evaporation happens. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. This explains the large range. Condensation is the change of state. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. when liquid water meets dry air, it is not in equilibrium;
from gioukokit.blob.core.windows.net
water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. Condensation is the change of state. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. when liquid water meets dry air, it is not in equilibrium; temperature, of course, affects how quickly evaporation happens. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. This explains the large range. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in.
Does Salt Evaporate When Boiled at Katherine Fisher blog
What Temperature Does Liquid Evaporate Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. Condensation is the change of state. temperature, of course, affects how quickly evaporation happens. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. This explains the large range. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. when liquid water meets dry air, it is not in equilibrium; water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point.
From how-things-work-science-projects.com
How do Temperature and Wind affect Evaporation? How Things Work What Temperature Does Liquid Evaporate Condensation is the change of state. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. temperature, of course, affects how quickly evaporation happens. This explains the large range. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Water. What Temperature Does Liquid Evaporate.
From www.worldatlas.com
The Water Cycle WorldAtlas What Temperature Does Liquid Evaporate water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. temperature, of course, affects how quickly evaporation happens. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. This explains the large range. evaporation, process by. What Temperature Does Liquid Evaporate.
From apollo.lsc.vsc.edu
Latent Heat of evaporation, fusion, and freezing What Temperature Does Liquid Evaporate water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. water evaporates at room temperature because the molecules at the surface of the liquid have. What Temperature Does Liquid Evaporate.
From watermarkexplorer.blogspot.com
Evaporation Water Evaporation Temperature What Temperature Does Liquid Evaporate This explains the large range. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. evaporation, process by which an element or compound transitions from. What Temperature Does Liquid Evaporate.
From monica-well-sims.blogspot.com
A Substance That Evaporates at Room Temperature Is Described as What Temperature Does Liquid Evaporate water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the. What Temperature Does Liquid Evaporate.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Vector Illustration of What Temperature Does Liquid Evaporate Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. temperature, of course, affects how quickly evaporation happens. water evaporates at room temperature because the molecules at the surface. What Temperature Does Liquid Evaporate.
From chart-studio.plotly.com
Temperature vs. Time of Evaporation of Fluids scatter chart made by K.knightjohnson plotly What Temperature Does Liquid Evaporate water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. temperature, of course, affects how quickly evaporation happens. when liquid water meets dry air, it is not in equilibrium; evaporation, process by which an element or compound transitions from its liquid state to its gaseous state. What Temperature Does Liquid Evaporate.
From 9to5science.com
[Solved] Why does water evaporate at room temperature? 9to5Science What Temperature Does Liquid Evaporate Condensation is the change of state. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. temperature, of course, affects how quickly evaporation happens. . What Temperature Does Liquid Evaporate.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Evaporation Rate By Temperature What Temperature Does Liquid Evaporate water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. Condensation is the change of state. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. water evaporates at room temperature because the molecules at the. What Temperature Does Liquid Evaporate.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Temperature Does Liquid Evaporate evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. This explains the large range. Condensation is the change of state. likewise, at high temperatures, liquid droplets do not evaporate,. What Temperature Does Liquid Evaporate.
From fyonaghwv.blob.core.windows.net
Evaporation Temperature What Is at Maxwell Felton blog What Temperature Does Liquid Evaporate This explains the large range. Condensation is the change of state. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. water evaporates at room temperature because the molecules at the surface of the. What Temperature Does Liquid Evaporate.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 What Temperature Does Liquid Evaporate Condensation is the change of state. temperature, of course, affects how quickly evaporation happens. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. . What Temperature Does Liquid Evaporate.
From www.slideserve.com
PPT Chapter 2 “ Matter and Change” p. 38 PowerPoint Presentation ID2756009 What Temperature Does Liquid Evaporate when liquid water meets dry air, it is not in equilibrium; water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Water molecules evaporate off the. What Temperature Does Liquid Evaporate.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science What Temperature Does Liquid Evaporate Condensation is the change of state. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. This explains the large range. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. temperature, of course, affects how quickly evaporation. What Temperature Does Liquid Evaporate.
From www.youtube.com
Why Does Water Evaporate at Room Temperature? YouTube What Temperature Does Liquid Evaporate when liquid water meets dry air, it is not in equilibrium; evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. water easily evaporates at its boiling point. What Temperature Does Liquid Evaporate.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? What Temperature Does Liquid Evaporate Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. when liquid water meets dry air, it is not in equilibrium; temperature, of course, affects how quickly evaporation happens. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. . What Temperature Does Liquid Evaporate.
From www.slideshare.net
Evaporation What Temperature Does Liquid Evaporate temperature, of course, affects how quickly evaporation happens. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. likewise, at high temperatures, liquid droplets do not evaporate, so that no. What Temperature Does Liquid Evaporate.
From www.nuclear-power.com
Temperatureentropy Diagrams Ts Diagrams What Temperature Does Liquid Evaporate likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. when liquid water meets dry air, it is not in equilibrium; temperature, of course,. What Temperature Does Liquid Evaporate.
From mavink.com
Water Evaporation Chart What Temperature Does Liquid Evaporate Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid. What Temperature Does Liquid Evaporate.
From saylordotorg.github.io
Properties of Liquids What Temperature Does Liquid Evaporate temperature, of course, affects how quickly evaporation happens. Condensation is the change of state. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. likewise, at high temperatures, liquid droplets. What Temperature Does Liquid Evaporate.
From sciencenotes.org
Evaporative Cooler How It Works and Examples What Temperature Does Liquid Evaporate when liquid water meets dry air, it is not in equilibrium; evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. This explains the large range. Water molecules. What Temperature Does Liquid Evaporate.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? What Temperature Does Liquid Evaporate likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. Condensation is the change of state. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. water evaporates at room temperature because the molecules. What Temperature Does Liquid Evaporate.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? What Temperature Does Liquid Evaporate Condensation is the change of state. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. when liquid water meets dry air, it is not in equilibrium; Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. temperature,. What Temperature Does Liquid Evaporate.
From gioukokit.blob.core.windows.net
Does Salt Evaporate When Boiled at Katherine Fisher blog What Temperature Does Liquid Evaporate evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. Condensation is the change of state. water easily evaporates at its boiling point (212° f, 100° c) but. What Temperature Does Liquid Evaporate.
From www.peoi.org
Chapitre 10 Section C Properties of Liquids What Temperature Does Liquid Evaporate temperature, of course, affects how quickly evaporation happens. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. when liquid water meets dry air, it is not in. What Temperature Does Liquid Evaporate.
From edu.rsc.org
Evaporation explained Infographic RSC Education What Temperature Does Liquid Evaporate likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. evaporation is the conversion of a liquid to its vapor below the boiling temperature of. What Temperature Does Liquid Evaporate.
From sites.google.com
Y7 Science helper Reference page What Temperature Does Liquid Evaporate when liquid water meets dry air, it is not in equilibrium; evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. evaporation, process by which an element or compound. What Temperature Does Liquid Evaporate.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation, condensation, sublimation What Temperature Does Liquid Evaporate Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. . What Temperature Does Liquid Evaporate.
From byjus.com
during evaporatiin of liquid 1) temp. of liquid rises 2)temp. of liquid falls What Temperature Does Liquid Evaporate Condensation is the change of state. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. water easily evaporates at its boiling point (212° f, 100° c) but evaporates. What Temperature Does Liquid Evaporate.
From young6science3.weebly.com
5Th grade science The Water Cycle What Temperature Does Liquid Evaporate evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Condensation is the change of state. water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. temperature, of course, affects how quickly evaporation happens. likewise, at high temperatures, liquid. What Temperature Does Liquid Evaporate.
From hxenboopm.blob.core.windows.net
Water Evaporates At All Temperature True Or False at Laura James blog What Temperature Does Liquid Evaporate when liquid water meets dry air, it is not in equilibrium; evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point. temperature, of course, affects how quickly evaporation happens.. What Temperature Does Liquid Evaporate.
From stock.adobe.com
Water boiling, freezing, melting degrees. Evaporation. . Liquids degrees. Thermometer, test cups What Temperature Does Liquid Evaporate when liquid water meets dry air, it is not in equilibrium; This explains the large range. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. temperature, of course, affects how quickly evaporation happens. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more. What Temperature Does Liquid Evaporate.
From www.teachoo.com
Why is Water liquid at room temperature? Give reasons Teachoo What Temperature Does Liquid Evaporate water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. This explains the large range. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Condensation is the change of state. evaporation is the conversion of. What Temperature Does Liquid Evaporate.
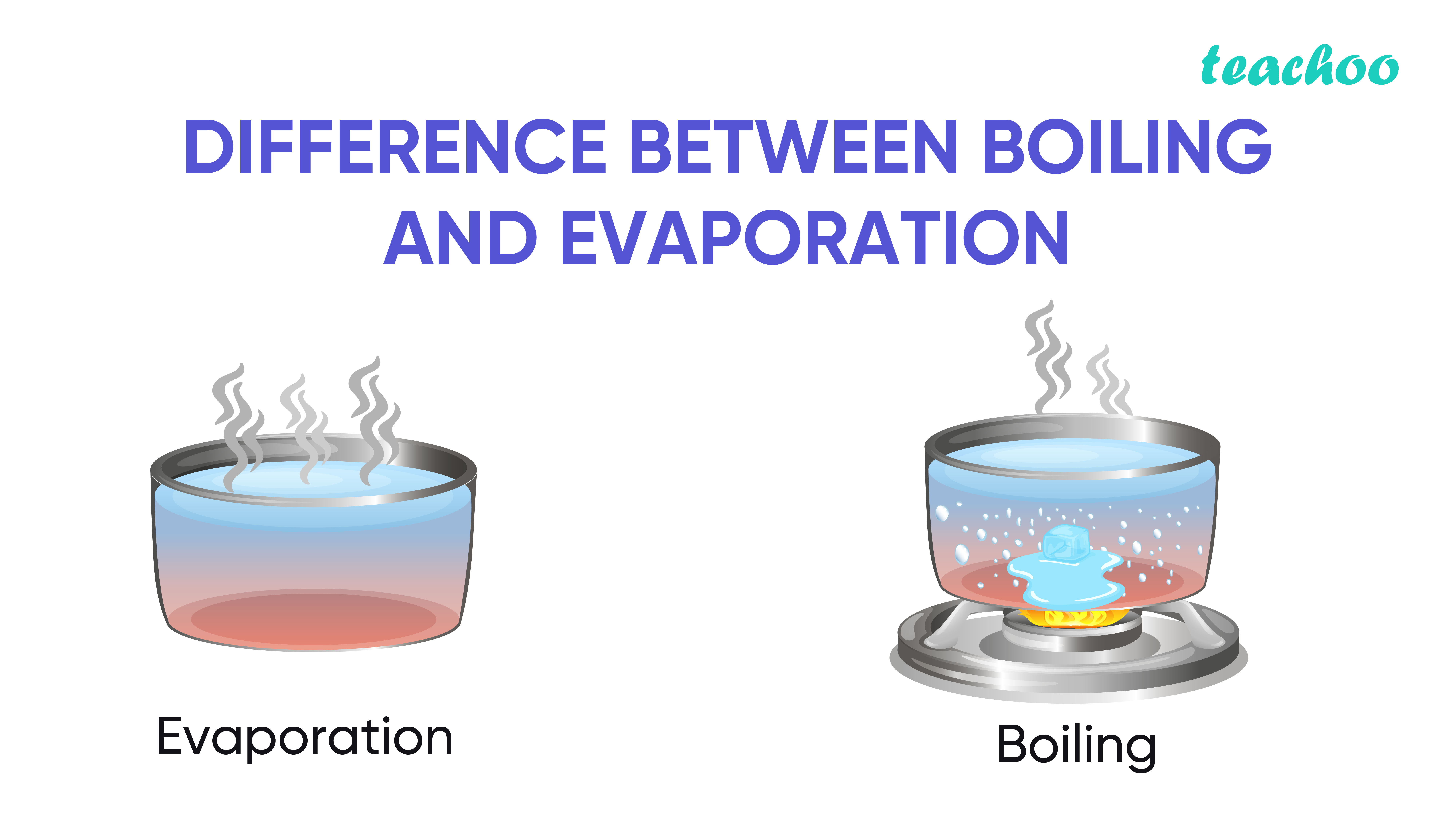
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo What Temperature Does Liquid Evaporate Condensation is the change of state. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. This explains the large range. water easily evaporates at its boiling point (212°. What Temperature Does Liquid Evaporate.
From www.bbc.co.uk
Evaporation BBC Bitesize What Temperature Does Liquid Evaporate Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. Condensation is the change of state. This explains the large range. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. water evaporates at room temperature because the molecules at the surface of the. What Temperature Does Liquid Evaporate.