Titration Buffers . In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Prepare and standardize a solution of sodium hydroxide. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Structures and properties, 2nd ed: A mixture of a weak acid and its conjugate base (or a mixture of a weak base. Buffers are characterized by their ph range and buffer capacity. Buffers and titrations ch16.1 buffer solutions. Select appropriate indicators for use in a titration.
from slidetodoc.com
A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Buffers and titrations ch16.1 buffer solutions. Buffers are characterized by their ph range and buffer capacity. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Select appropriate indicators for use in a titration. A mixture of a weak acid and its conjugate base (or a mixture of a weak base. Structures and properties, 2nd ed: Prepare and standardize a solution of sodium hydroxide. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration.
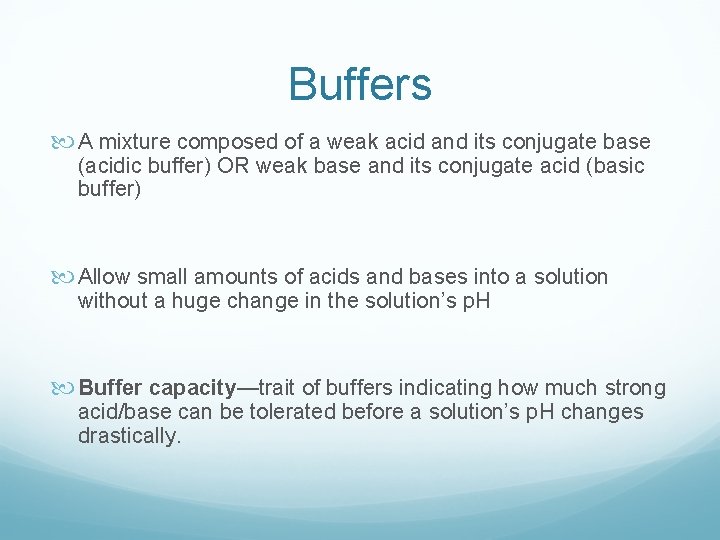
AcidBase Titration Buffers Buffers A mixture composed of
Titration Buffers A mixture of a weak acid and its conjugate base (or a mixture of a weak base. Select appropriate indicators for use in a titration. Prepare and standardize a solution of sodium hydroxide. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Buffers are characterized by their ph range and buffer capacity. Structures and properties, 2nd ed: A mixture of a weak acid and its conjugate base (or a mixture of a weak base. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Buffers and titrations ch16.1 buffer solutions. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration.
From favpng.com
Titration Curve Buffer Solution Acidbase Titration PH, PNG, 1024x721px Titration Buffers Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A chemical reaction is set up between a known volume of a solution of unknown concentration and a known. Titration Buffers.
From ar.inspiredpencil.com
Titration Curve Buffer Region Titration Buffers Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Structures and properties, 2nd ed: In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Prepare and standardize a solution of sodium hydroxide. Buffers are characterized by their ph. Titration Buffers.
From chart-studio.plotly.com
Titration of Phosphate Buffer with Base scatter chart made by Titration Buffers Structures and properties, 2nd ed: Buffers are characterized by their ph range and buffer capacity. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance. Titration Buffers.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner Titration Buffers Prepare and standardize a solution of sodium hydroxide. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Buffers are characterized by their ph range and buffer capacity. Buffers. Titration Buffers.
From www.scribd.com
Buffers and Titrations Post PDF Buffer Solution Titration Titration Buffers Select appropriate indicators for use in a titration. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A mixture of a weak acid and its conjugate base (or a mixture of a weak base. Titration is a procedure used in chemistry in order to determine the molarity. Titration Buffers.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Titration Buffers Structures and properties, 2nd ed: In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Buffers are characterized by their ph. Titration Buffers.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Buffers A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Prepare and standardize a solution of sodium hydroxide. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Structures and properties, 2nd ed: Buffers are characterized by. Titration Buffers.
From www.studypool.com
SOLUTION Lecture 7 titration buffers 1 Studypool Titration Buffers Prepare and standardize a solution of sodium hydroxide. A mixture of a weak acid and its conjugate base (or a mixture of a weak base. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Select appropriate indicators for use in a titration. Buffers are characterized by their. Titration Buffers.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Buffers A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Select appropriate indicators for use in a titration. A mixture of a weak acid and its conjugate. Titration Buffers.
From mavink.com
Titration Labeled Titration Buffers In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. A titration curve is a graph that relates the change in. Titration Buffers.
From www.animalia-life.club
Titration Curve Amino Acid Titration Buffers Buffers and titrations ch16.1 buffer solutions. Prepare and standardize a solution of sodium hydroxide. Buffers are characterized by their ph range and buffer capacity. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Structures and properties, 2nd ed: A chemical reaction is set up between. Titration Buffers.
From slidetodoc.com
AcidBase Titration Buffers Buffers A mixture composed of Titration Buffers A mixture of a weak acid and its conjugate base (or a mixture of a weak base. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Select appropriate indicators for use in a titration. Structures and properties, 2nd ed: Buffers are characterized by their ph range and. Titration Buffers.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Buffers Buffers are characterized by their ph range and buffer capacity. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Select appropriate indicators for use in a titration. Structures and properties, 2nd ed: Titration is a procedure used in chemistry in order to determine the molarity of an. Titration Buffers.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Titration Buffers Structures and properties, 2nd ed: Buffers are characterized by their ph range and buffer capacity. A mixture of a weak acid and its conjugate base (or a mixture of a weak base. Select appropriate indicators for use in a titration. Prepare and standardize a solution of sodium hydroxide. Titration is a procedure used in chemistry in order to determine the. Titration Buffers.
From forums.studentdoctor.net
Confusion about titration of polyprotic acids Student Doctor Network Titration Buffers Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base. Buffers are characterized by their ph range and buffer capacity. Structures and properties, 2nd ed: A chemical reaction is set up between a known. Titration Buffers.
From socratic.org
Is a solution of a weak acid considered a buffer solution? Socratic Titration Buffers A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Select appropriate indicators for use in a titration. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A mixture of a weak acid. Titration Buffers.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Buffers A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Prepare and standardize a solution of sodium hydroxide. Structures and properties, 2nd ed: In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A. Titration Buffers.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Buffers A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Prepare and standardize a solution of sodium hydroxide. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Structures and properties, 2nd ed: Select appropriate indicators for. Titration Buffers.
From www.elettronicaveneta.com
Buffers and titration curves Elettronica S.p.A. Titration Buffers In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Select appropriate indicators for use in a titration. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Buffers are characterized by their ph. Titration Buffers.
From mavink.com
Titration Labeled Titration Buffers Select appropriate indicators for use in a titration. A mixture of a weak acid and its conjugate base (or a mixture of a weak base. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A chemical reaction is set up between a known volume of a solution of unknown concentration. Titration Buffers.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Titration Buffers Buffers are characterized by their ph range and buffer capacity. Structures and properties, 2nd ed: In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A mixture of a weak acid and its conjugate base (or a mixture of a weak base. Titration is a procedure used in. Titration Buffers.
From www.youtube.com
titration with a buffer YouTube Titration Buffers Buffers are characterized by their ph range and buffer capacity. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Buffers and titrations ch16.1 buffer solutions. Structures and properties, 2nd ed: A titration curve is a graph that relates the change in ph of an acidic or basic solution to the. Titration Buffers.
From pdfslide.net
(PPTX) Buffers and Acid/Base Titration. Common Ion Suppose we have a Titration Buffers Select appropriate indicators for use in a titration. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Buffers and titrations ch16.1 buffer solutions. Prepare and standardize a solution of sodium hydroxide. A mixture of a weak acid and its conjugate base (or a mixture of a weak base. Structures and. Titration Buffers.
From www.studypool.com
SOLUTION Lecture 4 indicators and buffers and titrations Studypool Titration Buffers In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. A titration curve is a graph that relates the change in. Titration Buffers.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Buffers Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Prepare and standardize a solution of sodium hydroxide. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Buffers are characterized by their ph. Titration Buffers.
From byjus.com
Buffer Region What is a Buffer Region, Relationship between Titration Titration Buffers Select appropriate indicators for use in a titration. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base. Buffers are characterized by their ph range and buffer capacity. In a titration, a solution of. Titration Buffers.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Buffers A mixture of a weak acid and its conjugate base (or a mixture of a weak base. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Prepare and standardize a solution of sodium hydroxide. Structures and properties, 2nd ed: In a titration, a solution of. Titration Buffers.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Buffers Structures and properties, 2nd ed: Buffers and titrations ch16.1 buffer solutions. Prepare and standardize a solution of sodium hydroxide. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of. Titration Buffers.
From www.youtube.com
Buffers and Titration Curves YouTube Titration Buffers In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. A mixture of a weak acid and its conjugate base (or. Titration Buffers.
From www.pinterest.com
Pin em Biochemistry Titration Buffers A titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. Prepare and standardize a solution of sodium hydroxide. A mixture of a weak acid and its conjugate base (or a mixture of a weak base. A chemical reaction is set up between a known volume of. Titration Buffers.
From users.highland.edu
Buffers Titration Buffers Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Prepare and standardize a solution of sodium hydroxide. Buffers are characterized by their ph range and buffer capacity. Structures and properties, 2nd ed: A chemical reaction is set up between a known volume of a solution of unknown concentration and a. Titration Buffers.
From derangedphysiology.com
Buffers and buffering power Deranged Physiology Titration Buffers A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A mixture of a weak acid and its conjugate base (or a mixture of. Titration Buffers.
From pilgaard.info
Acids and bases Buffers Michael Pilgaard's Chemistry Titration Buffers Buffers and titrations ch16.1 buffer solutions. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Select appropriate indicators for use in a titration. A mixture of a weak acid and its conjugate base (or a mixture of a weak base. A chemical reaction is set up between. Titration Buffers.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Buffers A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Buffers are characterized by their ph range and buffer capacity. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Buffers and titrations ch16.1. Titration Buffers.
From www.vrogue.co
Top 10 Apparatus For Titration Of 2020 No Place Calle vrogue.co Titration Buffers Structures and properties, 2nd ed: Buffers and titrations ch16.1 buffer solutions. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. A mixture of a weak acid and its conjugate base (or a mixture of a weak base. Titration is a procedure used. Titration Buffers.