Example Titration Results Table . For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. If each subsequent k a is at least a 1000 times smaller than the previous. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. Titrating a weak acid with a strong base. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. This technique can be used to find: This results in a titration curve like that of figure 17.3.7. Concentration mr formula water of crystalisation. 9.2.2 selecting and evaluating the end point. To do this you react a. A student performs a titration experiment to determine the concentration of a solution of nitric acid.
from www.chegg.com
To do this you react a. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. Titrating a weak acid with a strong base. Concentration mr formula water of crystalisation. This results in a titration curve like that of figure 17.3.7. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. A student performs a titration experiment to determine the concentration of a solution of nitric acid. 9.2.2 selecting and evaluating the end point. This technique can be used to find: Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:.
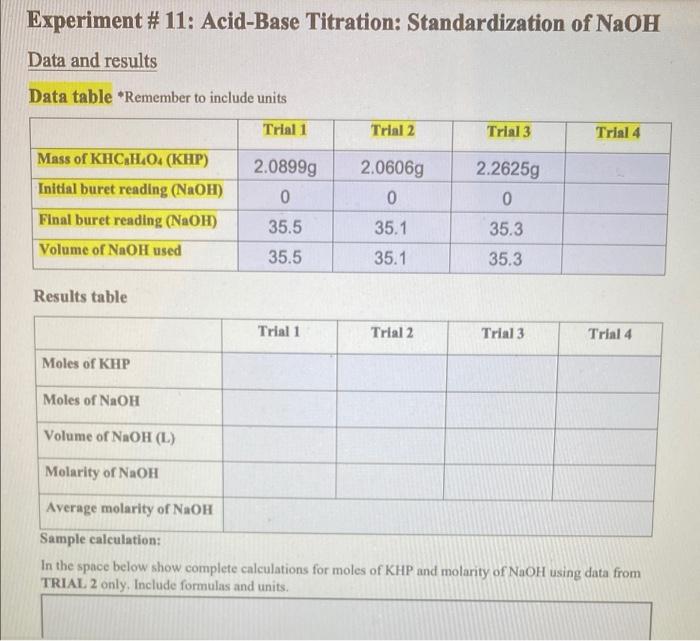
Solved Experiment 11 AcidBase Titration Standardization
Example Titration Results Table Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. 9.2.2 selecting and evaluating the end point. If each subsequent k a is at least a 1000 times smaller than the previous. Titrating a weak acid with a strong base. To do this you react a. This results in a titration curve like that of figure 17.3.7. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. This technique can be used to find: A student performs a titration experiment to determine the concentration of a solution of nitric acid. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. Concentration mr formula water of crystalisation. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e.
From www.chegg.com
Solved Data Table 2. Titration Curve values. Drops NaOH Example Titration Results Table Concentration mr formula water of crystalisation. To do this you react a. 9.2.2 selecting and evaluating the end point. If each subsequent k a is at least a 1000 times smaller than the previous. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. Recording results •results should be clearly recorded. Example Titration Results Table.
From www.slideshare.net
Titrations Example Titration Results Table To do this you react a. This technique can be used to find: Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. If each subsequent k a is at least a 1000 times smaller than the previous. Titrating a weak acid with a strong base. For each trial, 25.0 ml. Example Titration Results Table.
From www.chegg.com
Solved The following table shows the data for the titration Example Titration Results Table Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. This technique can be used to find: To do this you react a. This results in a titration curve like that of figure 17.3.7. Titrating a weak acid with a strong base. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein,. Example Titration Results Table.
From www.savemyexams.com
pH Titration Curves OCR A Level Chemistry Revision Notes 2017 Example Titration Results Table Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. 9.2.2 selecting and evaluating the end point. This technique can be used to find: If each subsequent k a is at least a 1000 times smaller than the previous. A student performs a titration experiment to determine the concentration of a. Example Titration Results Table.
From mstimms-btecmedicalscience12.blogspot.com
BTEC Medical science titration results table Example Titration Results Table A student performs a titration experiment to determine the concentration of a solution of nitric acid. 9.2.2 selecting and evaluating the end point. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. This. Example Titration Results Table.
From www.youtube.com
02 Experimental Set Up and Drawing Titration Table YouTube Example Titration Results Table If each subsequent k a is at least a 1000 times smaller than the previous. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. 9.2.2 selecting and evaluating the end point. This results in a titration curve like that of figure 17.3.7. This technique can be used to find: Titrating a weak. Example Titration Results Table.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Example Titration Results Table 9.2.2 selecting and evaluating the end point. A student performs a titration experiment to determine the concentration of a solution of nitric acid. This technique can be used to find: Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. This results in a titration curve like that of figure 17.3.7.. Example Titration Results Table.
From www.numerade.com
SOLVED 5 Use table to record your titration data in the data and observations section of your Example Titration Results Table A student performs a titration experiment to determine the concentration of a solution of nitric acid. Titrating a weak acid with a strong base. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. 9.2.2 selecting and evaluating the end point. To do this you react a. For each trial, 25.0 ml of. Example Titration Results Table.
From www.researchgate.net
Titration results of DEEA concentrations before and after the WWC... Download Table Example Titration Results Table This results in a titration curve like that of figure 17.3.7. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. To do this you react a. If each subsequent k a is at least a 1000 times smaller than the previous. This technique can be used to find: Concentration mr formula water of crystalisation.. Example Titration Results Table.
From www.coursehero.com
[Solved] The table given shows data from the titration of four 10.0 mL... Course Hero Example Titration Results Table 9.2.2 selecting and evaluating the end point. Titrating a weak acid with a strong base. Concentration mr formula water of crystalisation. If each subsequent k a is at least a 1000 times smaller than the previous. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. Given the titration of sulfuric acid with. Example Titration Results Table.
From www.researchgate.net
Screening results of various titrations Download Table Example Titration Results Table For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. Titrating a weak acid with a strong base. A student performs a titration experiment to determine the concentration of a solution of nitric acid. Concentration mr formula water. Example Titration Results Table.
From www.chegg.com
Solved Experiment 11 AcidBase Titration Standardization Example Titration Results Table 9.2.2 selecting and evaluating the end point. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. Concentration mr formula water of crystalisation. Titrating a weak acid with a strong base. If each subsequent k a is at least a 1000 times smaller than the previous. This technique can be used. Example Titration Results Table.
From www.chegg.com
Solved Results Titration Tables Table 5. Titration of Example Titration Results Table Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. This technique can be used to find: 9.2.2 selecting and evaluating the end point. A student performs a titration experiment to determine the concentration of a solution of nitric acid. Concentration mr formula water of crystalisation. If each subsequent k a is at. Example Titration Results Table.
From www.vrogue.co
Acid Base Titration Results vrogue.co Example Titration Results Table To do this you react a. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. If each subsequent k a is at least a 1000 times smaller than the previous. A student performs a titration experiment to determine the concentration of a solution of nitric acid. Titrating a weak acid with a strong base.. Example Titration Results Table.
From www.chegg.com
Solved Section Titration Lab Report Sheet Table 1 Acid Base Example Titration Results Table 9.2.2 selecting and evaluating the end point. If each subsequent k a is at least a 1000 times smaller than the previous. Concentration mr formula water of crystalisation. Titrating a weak acid with a strong base. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. To do this you react. Example Titration Results Table.
From www.studocu.com
Titration Lab Report Acid Base Titration Lab Report Table 3 NaOH molarity from titration Example Titration Results Table Concentration mr formula water of crystalisation. A student performs a titration experiment to determine the concentration of a solution of nitric acid. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. This technique can be used to find: Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested. Example Titration Results Table.
From www.youtube.com
Excel Tutorial 2 Titration Analysis YouTube Example Titration Results Table This technique can be used to find: For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. A student performs a titration experiment to determine the concentration of a solution of nitric acid. Concentration mr formula water of crystalisation. This results in a titration curve like that of figure 17.3.7. To do this you react. Example Titration Results Table.
From www.thestudentroom.co.uk
AS Chemistry Titration The Student Room Example Titration Results Table To do this you react a. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. 9.2.2 selecting and evaluating the end point. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. This technique can be used to find: Titrating a weak acid with a strong base.. Example Titration Results Table.
From www.studocu.com
Titration Report BTEC Applied Science Unit2 A Titration Report Introduction We have been Example Titration Results Table This results in a titration curve like that of figure 17.3.7. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. This technique can be used to find: Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. A student performs a titration experiment to determine. Example Titration Results Table.
From www.researchgate.net
Table of test results for potentiometric titration analysis. Download Table Example Titration Results Table To do this you react a. This results in a titration curve like that of figure 17.3.7. If each subsequent k a is at least a 1000 times smaller than the previous. A student performs a titration experiment to determine the concentration of a solution of nitric acid. Titrating a weak acid with a strong base. For each trial, 25.0. Example Titration Results Table.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 Determining the Concentration of Example Titration Results Table To do this you react a. Concentration mr formula water of crystalisation. This results in a titration curve like that of figure 17.3.7. 9.2.2 selecting and evaluating the end point. Titrating a weak acid with a strong base. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. A student performs a titration. Example Titration Results Table.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214 Example Titration Results Table Concentration mr formula water of crystalisation. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. To do this you react a. If each subsequent k a is at least a 1000 times smaller than the previous. Titrating a weak acid with a strong base. A student performs a titration experiment. Example Titration Results Table.
From www.hanlin.com
IB DP Chemistry SL复习笔记1.2.9 Titrations翰林国际教育 Example Titration Results Table Titrating a weak acid with a strong base. 9.2.2 selecting and evaluating the end point. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. This results in a titration curve like that of figure 17.3.7. If each subsequent k a is at least a 1000 times smaller than the previous. This technique can be. Example Titration Results Table.
From www.researchgate.net
UV/Vis DNA titration results Download Table Example Titration Results Table 9.2.2 selecting and evaluating the end point. If each subsequent k a is at least a 1000 times smaller than the previous. To do this you react a. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. Titrating a weak acid with a strong base. For each trial, 25.0 ml. Example Titration Results Table.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID4319437 Example Titration Results Table A student performs a titration experiment to determine the concentration of a solution of nitric acid. To do this you react a. This results in a titration curve like that of figure 17.3.7. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. Concentration mr formula water of crystalisation. Recording results. Example Titration Results Table.
From www.chegg.com
Solved Data Table 2 Titration Curve Values pH Value Trial 1 Example Titration Results Table Concentration mr formula water of crystalisation. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. A student performs a titration experiment to determine the concentration of a solution of nitric acid. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. 9.2.2 selecting. Example Titration Results Table.
From www.chegg.com
Table 2. Results of titration of EDTA with 0.025 M Example Titration Results Table Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. This results in a titration curve like that of figure 17.3.7. Titrating a weak acid with a strong base. 9.2.2 selecting and evaluating the end point. If each. Example Titration Results Table.
From keplarllp.com
️ Vitamin c redox titration. redox titration Essay. 20190218 Example Titration Results Table For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. 9.2.2 selecting and evaluating the end point. To do this you react a. If each subsequent k a is at least a 1000 times smaller than the previous. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e.. Example Titration Results Table.
From www.slideserve.com
PPT Acid Base Titration PowerPoint Presentation, free download ID803358 Example Titration Results Table Titrating a weak acid with a strong base. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. A student performs a titration experiment to determine the concentration of a solution of nitric acid. Concentration mr formula water of crystalisation. To do this you react a. This technique can be used to find: Given the. Example Titration Results Table.
From www.chegg.com
Titration Results Of 1/10 Dilution. Example Titration Results Table If each subsequent k a is at least a 1000 times smaller than the previous. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. This results in a titration curve like that of figure 17.3.7. Given the. Example Titration Results Table.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Example Titration Results Table Concentration mr formula water of crystalisation. If each subsequent k a is at least a 1000 times smaller than the previous. This results in a titration curve like that of figure 17.3.7. 9.2.2 selecting and evaluating the end point. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. Titrating a weak acid. Example Titration Results Table.
From www.researchgate.net
Titre values obtained from the visual titration for samples A to H. Download Table Example Titration Results Table Titrating a weak acid with a strong base. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. This technique can be used to find: Concentration mr formula water of crystalisation. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. To do this. Example Titration Results Table.
From mungfali.com
Titration Lab Diagram Example Titration Results Table A student performs a titration experiment to determine the concentration of a solution of nitric acid. This technique can be used to find: This results in a titration curve like that of figure 17.3.7. Concentration mr formula water of crystalisation. 9.2.2 selecting and evaluating the end point. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard. Example Titration Results Table.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Example Titration Results Table This results in a titration curve like that of figure 17.3.7. 9.2.2 selecting and evaluating the end point. Given the titration of sulfuric acid with sodium hydroxide, using phenolphthalein, here’s a suggested format for a results table:. Titrating a weak acid with a strong base. For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is.. Example Titration Results Table.
From www.chegg.com
Exercise 1 Back Titration of Antacid Neutralization Example Titration Results Table Concentration mr formula water of crystalisation. This technique can be used to find: For each trial, 25.0 ml of 0.050 mol/l of a sodium carbonate standard solution is. Recording results •results should be clearly recorded in a table •result should be recorded in full (i.e. A student performs a titration experiment to determine the concentration of a solution of nitric. Example Titration Results Table.