Neutralize Hcl . Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). Neutralization reactions are a specialized type. This neutralization, or titration can be expressed as follows: A reaction between an acid and a base is called a neutralization reaction and can be represented as: How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. Acid + base → h 2 o + salt. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used.
from www.numerade.com
The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. A reaction between an acid and a base is called a neutralization reaction and can be represented as: This neutralization, or titration can be expressed as follows: Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. Acid + base → h 2 o + salt. Neutralization reactions are a specialized type. How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?.
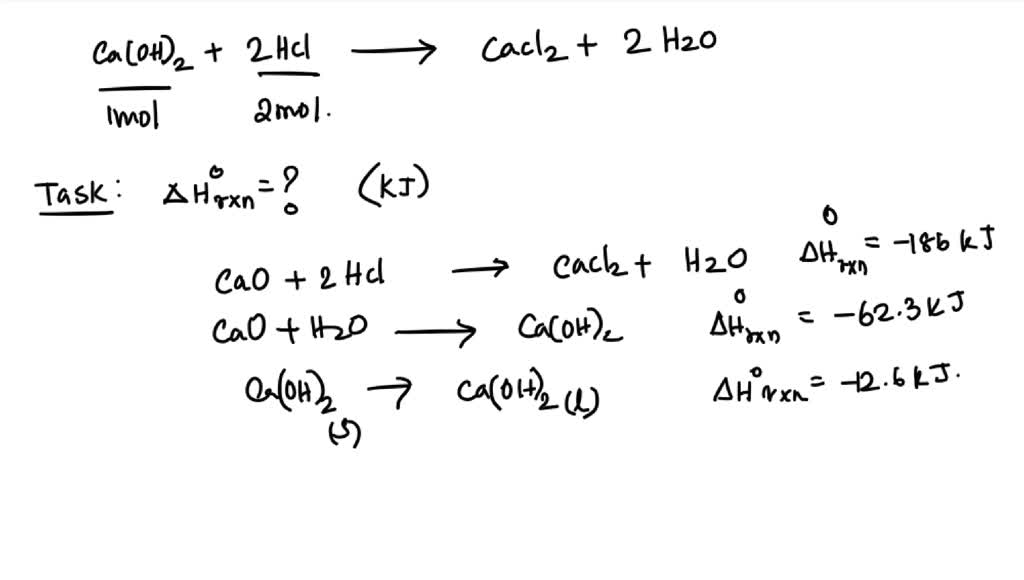
SOLVED 13 An aqueous solution of calcium hydroxide neutralizes
Neutralize Hcl Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. Neutralization reactions are a specialized type. This neutralization, or titration can be expressed as follows: Acid + base → h 2 o + salt. Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. A reaction between an acid and a base is called a neutralization reaction and can be represented as: When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used.
From www.chegg.com
Solved REVIEW QUESTIONS 1. a) What volume of 0.6 M HCl is Neutralize Hcl A reaction between an acid and a base is called a neutralization reaction and can be represented as: Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. Neutralization reactions are a specialized type. Hcl + naoh. Neutralize Hcl.
From fphoto.photoshelter.com
neutralization hydrochloric acid sodium hydroxide chemistry Neutralize Hcl This neutralization, or titration can be expressed as follows: Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. Acid + base → h 2 o + salt. How many moles of hydrochloric acid (hcl) would it take. Neutralize Hcl.
From dewwool.com
25 Examples of neutralization reaction DewWool Neutralize Hcl Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. A reaction between an acid and a base. Neutralize Hcl.
From www.pearson.com
A hydrochloric acid solution will neutralize a sodium hydroxide s Neutralize Hcl Neutralization reactions are a specialized type. Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. A reaction between an acid and a base is called a neutralization reaction and can be represented as: This neutralization, or titration can be expressed as follows: How many moles of hydrochloric acid (hcl) would it take to completely neutralize one. Neutralize Hcl.
From www.youtube.com
Acid/Base Neutralization Reaction for NaOH + HCl (Sodium hydroxide Neutralize Hcl When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). This neutralization, or titration can be expressed as follows: Acid +. Neutralize Hcl.
From www.chegg.com
Solved Antacids neutralize the hydrochloric acid in your Neutralize Hcl Acid + base → h 2 o + salt. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. This neutralization, or titration can be expressed as follows: Neutralization reactions are a specialized type. Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric. Neutralize Hcl.
From www.chegg.com
Solved A hydrochloric acid solution will neutralize a sodium Neutralize Hcl Neutralization reactions are a specialized type. Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. A reaction between an acid and a base is called a neutralization reaction and can be represented as: The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. Hcl +. Neutralize Hcl.
From www.dreamstime.com
Acidbase, Neutralization Reaction of Hydrochloric Acid and Sodium Neutralize Hcl Acid + base → h 2 o + salt. When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. A reaction between an acid and a base is called a neutralization reaction and can be represented as: Neutralization reactions are a specialized type. Write the balanced chemical equation for the neutralization. Neutralize Hcl.
From www.numerade.com
SOLVED 13 An aqueous solution of calcium hydroxide neutralizes Neutralize Hcl When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. This neutralization, or titration can be expressed as follows: The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. Write the balanced chemical equation for the neutralization of hcl with. Neutralize Hcl.
From plumbingarena.com
How To Neutralize Hydrochloric Acid? 8 Easy Hacks Neutralize Hcl A reaction between an acid and a base is called a neutralization reaction and can be represented as: Neutralization reactions are a specialized type. Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. This neutralization, or titration can be expressed as follows: The ph neutralization of hydrochloric acid is conventional and any inorganic base such as. Neutralize Hcl.
From www.reddit.com
What would you put in this trap to neutralize HCl gas created by Neutralize Hcl When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. This neutralization, or titration can be expressed as follows: Hcl + naoh → nacl + h 2 o in the. Neutralize Hcl.
From fphoto.photoshelter.com
neutralization hydrochloric acid sodium hydroxide chemistry Neutralize Hcl Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. A reaction between an acid and a base is called a neutralization reaction and can be represented as: Acid + base → h 2 o + salt. How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?.. Neutralize Hcl.
From fphoto.photoshelter.com
neutralization hydrochloric acid sodium hydroxide chemistry Neutralize Hcl How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. A reaction between an acid and a base is called a neutralization reaction and can be represented as: The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. Acid. Neutralize Hcl.
From www.slideserve.com
PPT Neutralization and Titrations! PowerPoint Presentation, free Neutralize Hcl How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). This neutralization, or titration can be expressed as follows: Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. Acid. Neutralize Hcl.
From solusgrp.com
How to Neutralize Hydrofluoric Acid (and Why You Can't Use Sulfuric Neutralize Hcl Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. A reaction between an acid and a base is. Neutralize Hcl.
From stock.adobe.com
Neutralization Reaction Infographic Diagram with example of Neutralize Hcl How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). A reaction. Neutralize Hcl.
From www.youtube.com
General Chemistry What volume of 0.50 M HCl is required to neutralize Neutralize Hcl Neutralization reactions are a specialized type. How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. Acid + base → h 2 o + salt. A reaction between an acid and a base is called a neutralization. Neutralize Hcl.
From sciencing.com
How to Use Baking Soda to Neutralize HCL Sciencing Neutralize Hcl When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. A reaction between an acid and a base is called a neutralization reaction and can be represented as: Write the. Neutralize Hcl.
From abigailkruwconner.blogspot.com
Enthalpy of Neutralization of Hcl and Naoh Value AbigailkruwConner Neutralize Hcl The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. A reaction between an acid and a base is called a neutralization reaction and can be represented as: Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). Write the balanced chemical. Neutralize Hcl.
From byjus.com
The enthalpy of neutralisation of HCl by NaOH is 57.1 kJ mol^ and that Neutralize Hcl Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). Acid + base → h 2 o + salt. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used.. Neutralize Hcl.
From sciencing.com
How to Use Baking Soda to Neutralize HCL Sciencing Neutralize Hcl Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. This neutralization, or titration can be expressed as follows: A reaction between an acid and a base is called a neutralization reaction and. Neutralize Hcl.
From study.com
Neutralizing Hydrochloric Acid Process & Calculations Lesson Neutralize Hcl Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. Neutralization reactions are a specialized type. Acid + base → h 2 o + salt. Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). A reaction between an acid and a base is called a neutralization reaction and can. Neutralize Hcl.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Neutralize Hcl Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. Acid + base → h 2 o + salt. This neutralization, or titration can be expressed as follows: The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. How many moles of hydrochloric acid (hcl) would. Neutralize Hcl.
From www.numerade.com
What volume of a 0.500 M HCl solution is needed to neutralize each of Neutralize Hcl Neutralization reactions are a specialized type. This neutralization, or titration can be expressed as follows: When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. Hcl + naoh → nacl. Neutralize Hcl.
From slideplayer.com
Neutralization of Strong Acids and Bases ppt download Neutralize Hcl Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. A reaction between an acid and a base is called a neutralization reaction and can be represented as: Neutralization reactions are a specialized type. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. When it. Neutralize Hcl.
From www.researchgate.net
4. Hydrochloric acid and sodium hydroxide neutralisation curves, [99 Neutralize Hcl Neutralization reactions are a specialized type. Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). Acid + base → h 2 o + salt. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. When it comes to excess inventory or. Neutralize Hcl.
From www.teachoo.com
Neutralization Reaction Definition, Equation and Examples Teachoo Neutralize Hcl The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. This neutralization, or titration can be expressed as follows: Neutralization reactions are a specialized type. A reaction between an. Neutralize Hcl.
From www.chegg.com
Solved 3. If it takes 25 mL of 0.05 M HCl to neutralize 345 Neutralize Hcl Acid + base → h 2 o + salt. Neutralization reactions are a specialized type. When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. The ph neutralization of hydrochloric acid is conventional and any inorganic base such. Neutralize Hcl.
From learningschooldihanieimy.z21.web.core.windows.net
Identify The Neutralization Reaction Neutralize Hcl The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. Acid + base → h 2 o + salt. Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. Neutralization reactions are a specialized type. Hcl + naoh → nacl + h 2 o in the. Neutralize Hcl.
From www.teachoo.com
Neutralization Reaction Definition, Equation and Examples Teachoo Neutralize Hcl When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. This neutralization, or titration can be expressed as follows: Hcl + naoh → nacl + h 2 o in the. Neutralize Hcl.
From googglet.com
Neutralization reaction between hcl and naoh Neutralize Hcl Neutralization reactions are a specialized type. How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior. Neutralize Hcl.
From www.youtube.com
Sodium hydroxide reaction with hydrochloric acid Neutralize Hcl Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). Neutralization reactions are a specialized type. Write the balanced chemical equation for the neutralization of hcl with mg(oh) 2. How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. The ph neutralization of. Neutralize Hcl.
From quizdbpharmacies.z4.web.core.windows.net
How To Neutralize Hcl Neutralize Hcl When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?. This neutralization, or titration can be expressed as follows: A reaction between an acid and a base is called a neutralization. Neutralize Hcl.
From sciencenotes.org
Neutralization Reaction Definition and Products Neutralize Hcl Acid + base → h 2 o + salt. A reaction between an acid and a base is called a neutralization reaction and can be represented as: Neutralization reactions are a specialized type. Hcl + naoh → nacl + h 2 o in the example shown above hydrochloric acid (hcl). When it comes to excess inventory or expired hydrochloric acid,. Neutralize Hcl.
From study.com
Neutralization Reaction Definition, Equation & Examples Lesson Neutralize Hcl When it comes to excess inventory or expired hydrochloric acid, proper neutralization is an essential step prior to disposal. The ph neutralization of hydrochloric acid is conventional and any inorganic base such as sodium hydroxide or lime can be used. How many moles of hydrochloric acid (hcl) would it take to completely neutralize one mole of aluminum hydroxide, al (oh)3?.. Neutralize Hcl.