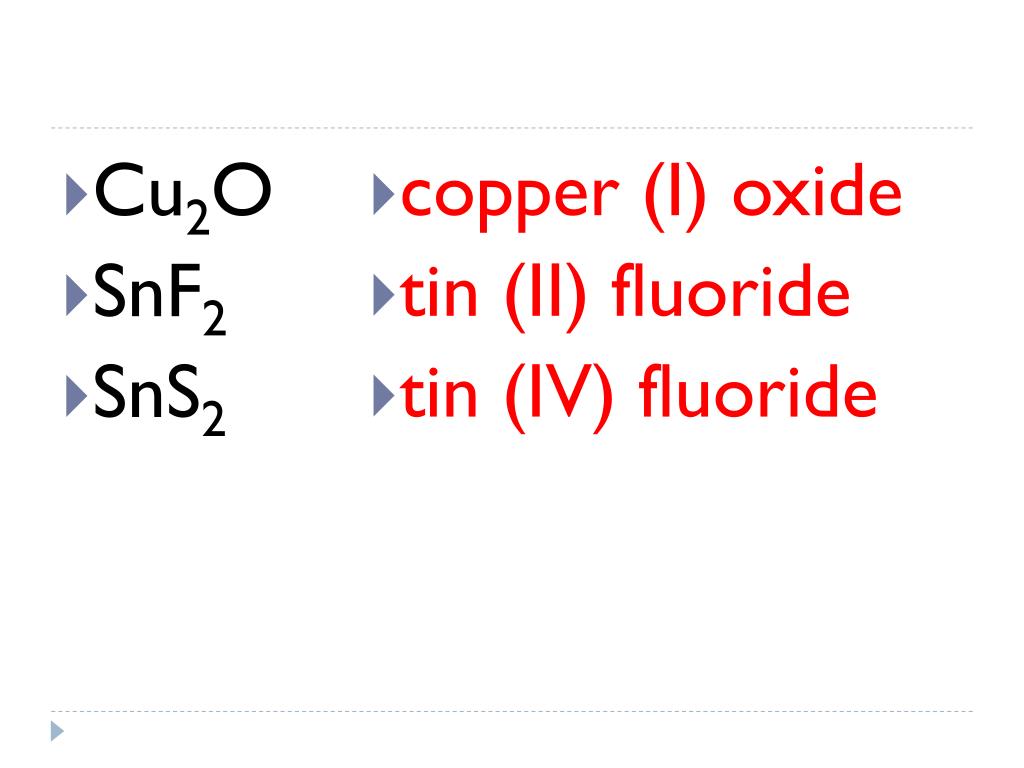
Tin Iv Sulfide Ionic Or Covalent . Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. Thus, the periodic table can. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Learn how to name ionic compounds using systematic and common names. The other fluoride of tin is snf 4, which was previously called. Define ionic and molecular (covalent) compounds. Predict the type of compound formed from elements based on their location within the. That is, does it have ionic bonds, or covalent bonds? The first question we ask is if the compound is ionic or covalent?
from www.slideserve.com
That is, does it have ionic bonds, or covalent bonds? This represents the formula snf 2, which is more properly named tin(ii) fluoride. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. Predict the type of compound formed from elements based on their location within the. The other fluoride of tin is snf 4, which was previously called. The first question we ask is if the compound is ionic or covalent? Define ionic and molecular (covalent) compounds. Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. Learn how to name ionic compounds using systematic and common names. Thus, the periodic table can.
PPT Naming Ions PowerPoint Presentation, free download ID4897123
Tin Iv Sulfide Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? The other fluoride of tin is snf 4, which was previously called. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Define ionic and molecular (covalent) compounds. Learn how to name ionic compounds using systematic and common names. The first question we ask is if the compound is ionic or covalent? Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. Thus, the periodic table can. That is, does it have ionic bonds, or covalent bonds? Predict the type of compound formed from elements based on their location within the.
From www.dreamstime.com
SnS2 Tin (IV) Sulfide stock image. Image of flask 296779805 Tin Iv Sulfide Ionic Or Covalent The other fluoride of tin is snf 4, which was previously called. Thus, the periodic table can. Predict the type of compound formed from elements based on their location within the. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Define. Tin Iv Sulfide Ionic Or Covalent.
From techiescientist.com
Is SiO2 Ionic or Covalent? Techiescientist Tin Iv Sulfide Ionic Or Covalent This represents the formula snf 2, which is more properly named tin(ii) fluoride. Thus, the periodic table can. The first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds? Define ionic and molecular (covalent) compounds. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Bonding PowerPoint Presentation, free download ID3995352 Tin Iv Sulfide Ionic Or Covalent This represents the formula snf 2, which is more properly named tin(ii) fluoride. Thus, the periodic table can. Learn how to name ionic compounds using systematic and common names. Define ionic and molecular (covalent) compounds. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. Find out how to identify the cation and the anion,. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT I. Ion Formation Ionic Formulas Ionic Nomenclature PowerPoint Tin Iv Sulfide Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. The first question we ask is if the compound is ionic or covalent? Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of. Tin Iv Sulfide Ionic Or Covalent.
From heegermaterials.com
Tin (IV) Sulfide SnS Heeger Materials Tin Iv Sulfide Ionic Or Covalent In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. Define ionic and molecular (covalent) compounds. The first question we ask is if the compound is ionic or covalent? Learn how to name ionic compounds using systematic and common names. The other fluoride of tin is snf 4, which was previously called. Thus, the periodic. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Calcium Sulfide PowerPoint Presentation, free download ID3730769 Tin Iv Sulfide Ionic Or Covalent Thus, the periodic table can. Predict the type of compound formed from elements based on their location within the. Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. Define ionic and molecular (covalent) compounds. Learn how to name ionic compounds using systematic and common names. This represents the. Tin Iv Sulfide Ionic Or Covalent.
From www.numerade.com
SOLVEDClassify each of the following solid elements as molecular Tin Iv Sulfide Ionic Or Covalent Predict the type of compound formed from elements based on their location within the. Thus, the periodic table can. Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. Learn how to name ionic compounds using systematic and common names. Define ionic and molecular (covalent) compounds. This represents the. Tin Iv Sulfide Ionic Or Covalent.
From www.chegg.com
Solved Tin(IV) sulfide, SnS2, a yellow pigment, can Tin Iv Sulfide Ionic Or Covalent Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. That is, does it have ionic bonds, or covalent bonds? Predict the type of compound formed from elements based on their location within the. The first question we ask is if the compound is ionic or covalent? Define ionic. Tin Iv Sulfide Ionic Or Covalent.
From www.slideshare.net
Ionic bonding binary Tin Iv Sulfide Ionic Or Covalent Define ionic and molecular (covalent) compounds. Predict the type of compound formed from elements based on their location within the. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. In this video we'll. Tin Iv Sulfide Ionic Or Covalent.
From www.numerade.com
SOLVEDKnowing that aqueous tin(IV) nitrate is mixed with H2S (aq) and Tin Iv Sulfide Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. The first question we ask is if the compound is ionic or covalent? Thus, the periodic table can. Define ionic and molecular (covalent) compounds. The other fluoride of tin is snf 4, which was previously. Tin Iv Sulfide Ionic Or Covalent.
From slideplayer.com
Ionic & Covalent Review ppt download Tin Iv Sulfide Ionic Or Covalent Predict the type of compound formed from elements based on their location within the. The first question we ask is if the compound is ionic or covalent? Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. Learn how to name ionic compounds using systematic and common. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Naming Ionic Compounds Wkst 1 PowerPoint Presentation, free Tin Iv Sulfide Ionic Or Covalent Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. Learn how to name ionic compounds using systematic and common names. Thus, the periodic table can. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. This represents the formula snf 2,. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Naming Ionic and covalent compounds PowerPoint Presentation, free Tin Iv Sulfide Ionic Or Covalent Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. Predict the type of compound formed from elements based on their location within the. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. Whereas ionic compounds are usually formed when a metal and. Tin Iv Sulfide Ionic Or Covalent.
From www.youtube.com
Is H2S Ionic or Covalent? (Hydrogen Sulfide) YouTube Tin Iv Sulfide Ionic Or Covalent Learn how to name ionic compounds using systematic and common names. That is, does it have ionic bonds, or covalent bonds? Predict the type of compound formed from elements based on their location within the. Thus, the periodic table can. This represents the formula snf 2, which is more properly named tin(ii) fluoride. The other fluoride of tin is snf. Tin Iv Sulfide Ionic Or Covalent.
From www.ossila.com
SnS2, Tin(IV) Sulfide Powder & Crystal CAS Number 1315011 Ossila Tin Iv Sulfide Ionic Or Covalent The first question we ask is if the compound is ionic or covalent? Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. That is, does it have ionic bonds, or covalent bonds? Thus, the periodic table can. This represents the formula snf 2, which is more. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID3730496 Tin Iv Sulfide Ionic Or Covalent Learn how to name ionic compounds using systematic and common names. The first question we ask is if the compound is ionic or covalent? Define ionic and molecular (covalent) compounds. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Thus, the periodic table can. That is, does it have ionic bonds, or covalent bonds? In this. Tin Iv Sulfide Ionic Or Covalent.
From slideplayer.com
Simple Chemical Nomenclature ppt download Tin Iv Sulfide Ionic Or Covalent Thus, the periodic table can. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. Predict the type of compound formed from elements based on their location within the. Find out how to identify the cation and the anion, and how to use roman numerals and suffixes. Tin Iv Sulfide Ionic Or Covalent.
From www.youtube.com
How to write chemical formula Tin IV Oxide YouTube Tin Iv Sulfide Ionic Or Covalent This represents the formula snf 2, which is more properly named tin(ii) fluoride. The first question we ask is if the compound is ionic or covalent? Learn how to name ionic compounds using systematic and common names. Thus, the periodic table can. Define ionic and molecular (covalent) compounds. In this video we'll write the correct formula for tin (iv) sulfide. Tin Iv Sulfide Ionic Or Covalent.
From www.pinterest.com
Difference Between Covalent and Ionic Bonds Chemistry education Tin Iv Sulfide Ionic Or Covalent Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. Predict the type of compound formed from elements based on their location within the. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. Learn how to name ionic compounds using systematic and common. Tin Iv Sulfide Ionic Or Covalent.
From www.youtube.com
Is SF4 (Sulfur tetrafluoride) Ionic or Covalent/Molecular? YouTube Tin Iv Sulfide Ionic Or Covalent In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. The first question we ask is if the compound is ionic or covalent? Predict the type of compound formed from elements based on their location within the. Find out how to identify the cation and the anion, and how to use roman numerals and suffixes. Tin Iv Sulfide Ionic Or Covalent.
From slideplayer.com
Chemical Nomenclature ppt download Tin Iv Sulfide Ionic Or Covalent Predict the type of compound formed from elements based on their location within the. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. Define ionic and molecular (covalent) compounds. That is, does it have ionic bonds, or covalent bonds? The first question we ask is if the compound is ionic or covalent? Whereas ionic. Tin Iv Sulfide Ionic Or Covalent.
From pnghero.com
Tiniv Sulfide Tin Bromide Lewis Structure Tin(IV) Oxide Structural Tin Iv Sulfide Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? The first question we ask is if the compound is ionic or covalent? Define ionic and molecular (covalent) compounds. Thus, the periodic table can. Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. Whereas ionic compounds are usually. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Nomenclature PowerPoint Presentation, free download ID1931353 Tin Iv Sulfide Ionic Or Covalent Learn how to name ionic compounds using systematic and common names. Predict the type of compound formed from elements based on their location within the. The first question we ask is if the compound is ionic or covalent? Thus, the periodic table can. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. This represents. Tin Iv Sulfide Ionic Or Covalent.
From studylib.net
Ionic & Covalent Nomenclature PowerPoint Tin Iv Sulfide Ionic Or Covalent This represents the formula snf 2, which is more properly named tin(ii) fluoride. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. The first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds? Whereas ionic compounds are usually formed when a metal. Tin Iv Sulfide Ionic Or Covalent.
From www.youtube.com
How to write the formula for tin (IV) chloride YouTube Tin Iv Sulfide Ionic Or Covalent Thus, the periodic table can. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. The first question we ask is if the compound is ionic. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Ch. 15 and 6 Naming and Writing Formulas for Ionic Compounds Tin Iv Sulfide Ionic Or Covalent The first question we ask is if the compound is ionic or covalent? This represents the formula snf 2, which is more properly named tin(ii) fluoride. Thus, the periodic table can. Define ionic and molecular (covalent) compounds. That is, does it have ionic bonds, or covalent bonds? Learn how to name ionic compounds using systematic and common names. The other. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Chemistry Nomenclature PowerPoint Presentation, free download Tin Iv Sulfide Ionic Or Covalent Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. This represents the formula snf 2, which is more properly named tin(ii) fluoride. That is, does it have ionic bonds, or covalent bonds? The other fluoride of tin is snf 4, which was previously called. Define ionic and molecular. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Ch. 15 and 6 Naming and Writing Formulas for Ionic Compounds Tin Iv Sulfide Ionic Or Covalent Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. That is, does it have ionic bonds, or covalent bonds? Learn how to name ionic compounds using systematic and common names. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed. Tin Iv Sulfide Ionic Or Covalent.
From www.nanochemazone.com
Tin(IV) Sulfide Powder Low Price 1 highly pure Nanochemazone Tin Iv Sulfide Ionic Or Covalent In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. Thus, the periodic table can. Predict the type of compound formed from elements based on their location within the. Whereas ionic compounds are usually. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Naming Ionic Compounds Wkst 1 PowerPoint Presentation ID1932060 Tin Iv Sulfide Ionic Or Covalent Predict the type of compound formed from elements based on their location within the. The other fluoride of tin is snf 4, which was previously called. Define ionic and molecular (covalent) compounds. That is, does it have ionic bonds, or covalent bonds? In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. Whereas ionic compounds. Tin Iv Sulfide Ionic Or Covalent.
From www.youtube.com
How to Write the Formula for Tin (IV) sulfide YouTube Tin Iv Sulfide Ionic Or Covalent Define ionic and molecular (covalent) compounds. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Thus, the periodic table can. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. Predict the type of compound formed from elements based on their location within the. Learn how to name ionic compounds using. Tin Iv Sulfide Ionic Or Covalent.
From slideplayer.com
Naming Ionic Compounds ppt download Tin Iv Sulfide Ionic Or Covalent This represents the formula snf 2, which is more properly named tin(ii) fluoride. The other fluoride of tin is snf 4, which was previously called. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. Learn how to name ionic compounds using systematic and common names. Find. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Tin Iv Sulfide Ionic Or Covalent The first question we ask is if the compound is ionic or covalent? The other fluoride of tin is snf 4, which was previously called. Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Define. Tin Iv Sulfide Ionic Or Covalent.
From topblogtenz.com
Is H2S ionic or covalent or both? Type of bond in Hydrogen sulfide? Tin Iv Sulfide Ionic Or Covalent Learn how to name ionic compounds using systematic and common names. In this video we'll write the correct formula for tin (iv) sulfide (sns2).to write the. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Define ionic and molecular (covalent) compounds. The first question we ask is if the compound is ionic or covalent? Find out. Tin Iv Sulfide Ionic Or Covalent.
From www.slideserve.com
PPT Naming Ions PowerPoint Presentation, free download ID4897123 Tin Iv Sulfide Ionic Or Covalent Predict the type of compound formed from elements based on their location within the. Find out how to identify the cation and the anion, and how to use roman numerals and suffixes for metals that. Thus, the periodic table can. This represents the formula snf 2, which is more properly named tin(ii) fluoride. That is, does it have ionic bonds,. Tin Iv Sulfide Ionic Or Covalent.