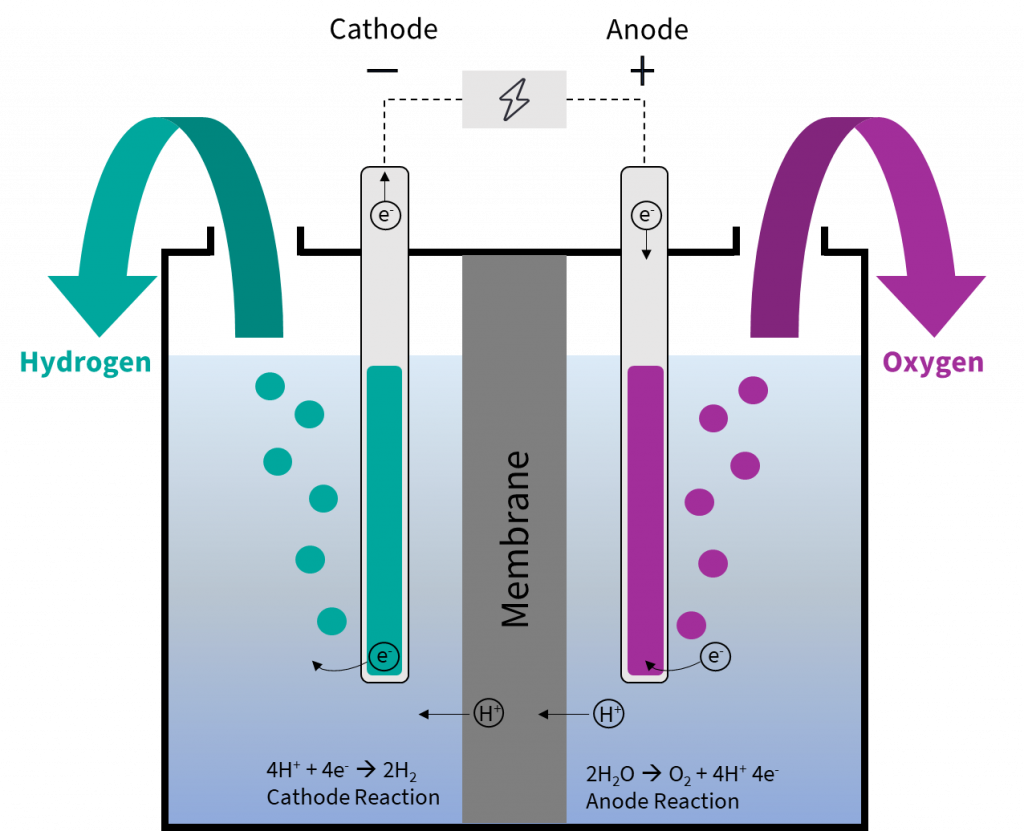
Electrolysis Electrode Rules . Using electricity to force a nonspontaneous process to occur is electrolysis. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. This page introduces the key words and ideas in electrolysis. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Ionic compounds conduct electricity when molten or in solution. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. You will find detailed examples of electrolysis in. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Electrolysis can also be used to produce h 2 and o 2 from water. Electrolyte is the ionic compound in. Reactive metals are extracted from their ores using electrolysis. The magnitude of charge (= 96488 c moh) is called faraday constant and is. Electrolytic cells are electrochemical cells with.
from ptx-hub.org
The magnitude of charge (= 96488 c moh) is called faraday constant and is. Electrolysis can also be used to produce h 2 and o 2 from water. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolytic cells are electrochemical cells with. Electrolyte is the ionic compound in. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. You will find detailed examples of electrolysis in. Using electricity to force a nonspontaneous process to occur is electrolysis.
Water electrolysis explained the basis for most PowertoX processes PtX Hub
Electrolysis Electrode Rules In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolytic cells are electrochemical cells with. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Using electricity to force a nonspontaneous process to occur is electrolysis. This page introduces the key words and ideas in electrolysis. Ionic compounds conduct electricity when molten or in solution. The magnitude of charge (= 96488 c moh) is called faraday constant and is. Electrolyte is the ionic compound in. Electrolysis can also be used to produce h 2 and o 2 from water. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Reactive metals are extracted from their ores using electrolysis. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. You will find detailed examples of electrolysis in.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download ID5285503 Electrolysis Electrode Rules Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. The magnitude of charge (= 96488 c moh) is called faraday constant and is. Using electricity to force a nonspontaneous process to occur is electrolysis. Ionic compounds conduct electricity when molten or in. Electrolysis Electrode Rules.
From en.ppt-online.org
Electrolysis online presentation Electrolysis Electrode Rules The magnitude of charge (= 96488 c moh) is called faraday constant and is. Ionic compounds conduct electricity when molten or in solution. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Electrolysis can. Electrolysis Electrode Rules.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolysis Electrode Rules The magnitude of charge (= 96488 c moh) is called faraday constant and is. This page introduces the key words and ideas in electrolysis. Electrolytic cells are electrochemical cells with. Electrolysis can also be used to produce h 2 and o 2 from water. Electrolyte is the ionic compound in. Learn about and revise electrolysis with this bbc bitesize gcse. Electrolysis Electrode Rules.
From en.ppt-online.org
Electrolysis online presentation Electrolysis Electrode Rules You will find detailed examples of electrolysis in. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolytic cells are electrochemical cells with. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Electrode is a rod of metal or graphite through which an electric current flows into or out of an. Electrolysis Electrode Rules.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Electrolysis Electrode Rules Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Using electricity to force a nonspontaneous process to occur is electrolysis. You will find detailed examples of electrolysis in. Reactive metals are extracted from their ores using electrolysis. Electrode is a rod of metal or graphite through which an electric current flows into or out. Electrolysis Electrode Rules.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Electrolysis Electrode Rules Electrolysis can also be used to produce h 2 and o 2 from water. Using electricity to force a nonspontaneous process to occur is electrolysis. The magnitude of charge (= 96488 c moh) is called faraday constant and is. Electrolyte is the ionic compound in. You will find detailed examples of electrolysis in. Electrolytic cells are electrochemical cells with. Reactive. Electrolysis Electrode Rules.
From www.onlinemathlearning.com
Chemical Reactions IGCSE Chemistry (solutions, examples, worksheets, videos) Electrolysis Electrode Rules Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. You will find detailed examples of electrolysis in. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. This page introduces the key words and ideas in electrolysis. Electrolysis can also be used to produce h 2 and o 2. Electrolysis Electrode Rules.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrolysis Electrode Rules Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Using electricity to force a nonspontaneous process to occur is electrolysis. Electrolysis can also be used to produce h 2 and o 2 from water.. Electrolysis Electrode Rules.
From www.elevise.co.uk
C4 L) Electrolysis Part 2 AQA Chemistry Elevise Electrolysis Electrode Rules In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa). Electrolysis Electrode Rules.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube Electrolysis Electrode Rules The magnitude of charge (= 96488 c moh) is called faraday constant and is. You will find detailed examples of electrolysis in. Ionic compounds conduct electricity when molten or in solution. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Learn about faraday's laws of electrolysis, the first law. Electrolysis Electrode Rules.
From www.tes.com
SC6.4 Electrolysis of aqueous solutions (AQA 91 GCSE Chemistry) Teaching Resources Electrolysis Electrode Rules Electrolysis can also be used to produce h 2 and o 2 from water. Using electricity to force a nonspontaneous process to occur is electrolysis. You will find detailed examples of electrolysis in. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Electrolytic cells are electrochemical cells with. Reactive metals are extracted from their. Electrolysis Electrode Rules.
From chemistnotes.com
Faraday's law of electrolysis First and Second law Chemistry Notes Electrolysis Electrode Rules You will find detailed examples of electrolysis in. This page introduces the key words and ideas in electrolysis. Electrolyte is the ionic compound in. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Using. Electrolysis Electrode Rules.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolysis Electrode Rules You will find detailed examples of electrolysis in. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Electrolysis can also be used to produce h 2 and o 2 from water. Reactive metals are extracted from their ores using electrolysis. This page introduces the key words and ideas in electrolysis. Learn about faraday's laws. Electrolysis Electrode Rules.
From www.snexplores.org
Explainer What is an electrode? Electrolysis Electrode Rules Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. The magnitude of charge (= 96488 c moh) is called faraday constant and is. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to produce h 2 and o 2 from water.. Electrolysis Electrode Rules.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? Electrolysis Electrode Rules Electrolyte is the ionic compound in. The magnitude of charge (= 96488 c moh) is called faraday constant and is. Ionic compounds conduct electricity when molten or in solution. You will find detailed examples of electrolysis in. This page introduces the key words and ideas in electrolysis. Using electricity to force a nonspontaneous process to occur is electrolysis. Electrode is. Electrolysis Electrode Rules.
From www.youtube.com
Electrolysis of Aqueous Solutions for AQA GCSE Chemistry YouTube Electrolysis Electrode Rules Ionic compounds conduct electricity when molten or in solution. Using electricity to force a nonspontaneous process to occur is electrolysis. This page introduces the key words and ideas in electrolysis. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Reactive metals are. Electrolysis Electrode Rules.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte, electrode, anode and cathode Electrolysis Electrode Rules Reactive metals are extracted from their ores using electrolysis. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Ionic compounds conduct electricity when molten or in solution. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Learn about and revise electrolysis with this. Electrolysis Electrode Rules.
From www.slideserve.com
PPT Lecture 2. Basic Electrochemistry PowerPoint Presentation, free download ID2281219 Electrolysis Electrode Rules Ionic compounds conduct electricity when molten or in solution. This page introduces the key words and ideas in electrolysis. Using electricity to force a nonspontaneous process to occur is electrolysis. Electrolysis can also be used to produce h 2 and o 2 from water. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. In. Electrolysis Electrode Rules.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Electrolysis Electrode Rules This page introduces the key words and ideas in electrolysis. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. Electrolytic cells are electrochemical cells with. Electrolysis can also be used to produce h 2 and o 2 from water. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction.. Electrolysis Electrode Rules.
From study.com
Faraday's Laws of Electrolysis Definition & Equation Video & Lesson Transcript Electrolysis Electrode Rules Electrolysis can also be used to produce h 2 and o 2 from water. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. This page introduces the key words and ideas in electrolysis. You will find detailed examples of electrolysis in. Electrolyte is the ionic compound in. Ionic compounds conduct electricity when molten or. Electrolysis Electrode Rules.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID4966899 Electrolysis Electrode Rules Electrolyte is the ionic compound in. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to produce h 2 and o 2 from water. This page introduces the key words and ideas in electrolysis. Reactive metals are. Electrolysis Electrode Rules.
From forestparkgolfcourse.com
Water Electrolysis Principle of Water Electrolysis, Important Factors, Electrolytes, (2023) Electrolysis Electrode Rules Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. Electrolysis can also be used to produce h 2 and o 2 from water. You will find detailed examples of electrolysis in. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Reactive metals are. Electrolysis Electrode Rules.
From www.youtube.com
Electrolysis Preferential discharge 2 (nature of electrode) YouTube Electrolysis Electrode Rules This page introduces the key words and ideas in electrolysis. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Electrolyte is the ionic compound in. The magnitude of charge (= 96488 c moh) is called faraday constant and is. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide.. Electrolysis Electrode Rules.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID5813936 Electrolysis Electrode Rules Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Reactive metals are extracted from their ores using electrolysis. Electrolytic cells are electrochemical cells with. Using electricity to force a nonspontaneous process to occur is electrolysis. This page introduces the key words and ideas in electrolysis. Electrode is a rod of metal or graphite through. Electrolysis Electrode Rules.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes PtX Hub Electrolysis Electrode Rules Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Reactive metals are extracted from their ores using electrolysis. Electrolyte is the ionic compound in. Ionic compounds conduct electricity when molten or in solution. You will find detailed examples of. Electrolysis Electrode Rules.
From chemrevisionigcse.blogspot.com
IGCSE Chemistry Electrolysis Electrolysis Electrode Rules Electrolyte is the ionic compound in. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to produce h 2 and o 2 from water. Reactive metals are extracted from their ores using electrolysis. Electrolytic cells are electrochemical cells with. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law. Electrolysis Electrode Rules.
From classnotes.gidemy.com
Electrochemistry Gidemy Class Notes Electrolysis Electrode Rules Using electricity to force a nonspontaneous process to occur is electrolysis. Electrolysis can also be used to produce h 2 and o 2 from water. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Reactive metals are extracted from their ores using electrolysis. Learn about and revise electrolysis with this bbc bitesize gcse combined. Electrolysis Electrode Rules.
From en.ppt-online.org
Electrolysis online presentation Electrolysis Electrode Rules Ionic compounds conduct electricity when molten or in solution. Using electricity to force a nonspontaneous process to occur is electrolysis. You will find detailed examples of electrolysis in. The magnitude of charge (= 96488 c moh) is called faraday constant and is. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Electrolytic cells are. Electrolysis Electrode Rules.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrolysis Electrode Rules Electrolyte is the ionic compound in. The magnitude of charge (= 96488 c moh) is called faraday constant and is. Electrolysis can also be used to produce h 2 and o 2 from water. Ionic compounds conduct electricity when molten or in solution. You will find detailed examples of electrolysis in. Reactive metals are extracted from their ores using electrolysis.. Electrolysis Electrode Rules.
From www.slideshare.net
IGCSE Electricity Electrolysis Electrode Rules Electrolyte is the ionic compound in. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. This page introduces the key words and ideas in electrolysis. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when. Electrolysis Electrode Rules.
From www.youtube.com
2 Electrolysis using Standard Electrode Potentials YouTube Electrolysis Electrode Rules Electrolytic cells are electrochemical cells with. Reactive metals are extracted from their ores using electrolysis. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. This page introduces the key words and ideas in electrolysis. Electrode is a rod of. Electrolysis Electrode Rules.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Electrolysis Electrode Rules This page introduces the key words and ideas in electrolysis. Electrolyte is the ionic compound in. Learn about and revise electrolysis with this bbc bitesize gcse combined science (aqa) study guide. Reactive metals are extracted from their ores using electrolysis. Learn about faraday's laws of electrolysis, the first law of electrolysis, second law of electrolysis. Electrolytic cells are electrochemical cells. Electrolysis Electrode Rules.
From study.com
Electrolysis of Aqueous Solutions Lesson Electrolysis Electrode Rules Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. The magnitude of charge (= 96488 c moh) is called faraday constant and is. Ionic compounds conduct electricity when molten or in solution. Electrolytic cells are electrochemical cells. Electrolysis Electrode Rules.
From www.slideserve.com
PPT 17.78 Electrolysis & Applications PowerPoint Presentation ID4499709 Electrolysis Electrode Rules This page introduces the key words and ideas in electrolysis. You will find detailed examples of electrolysis in. Electrode is a rod of metal or graphite through which an electric current flows into or out of an electrolyte. The magnitude of charge (= 96488 c moh) is called faraday constant and is. Ionic compounds conduct electricity when molten or in. Electrolysis Electrode Rules.
From www.tes.com
New GCSE Chemistry C6 Electrolysis lesson 1 introduction to electrolysis Teaching Resources Electrolysis Electrode Rules Electrolyte is the ionic compound in. Ionic compounds conduct electricity when molten or in solution. In electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to produce h 2 and o 2 from water. Electrode is a rod of metal or graphite through which an electric current flows into or out of an. Electrolysis Electrode Rules.