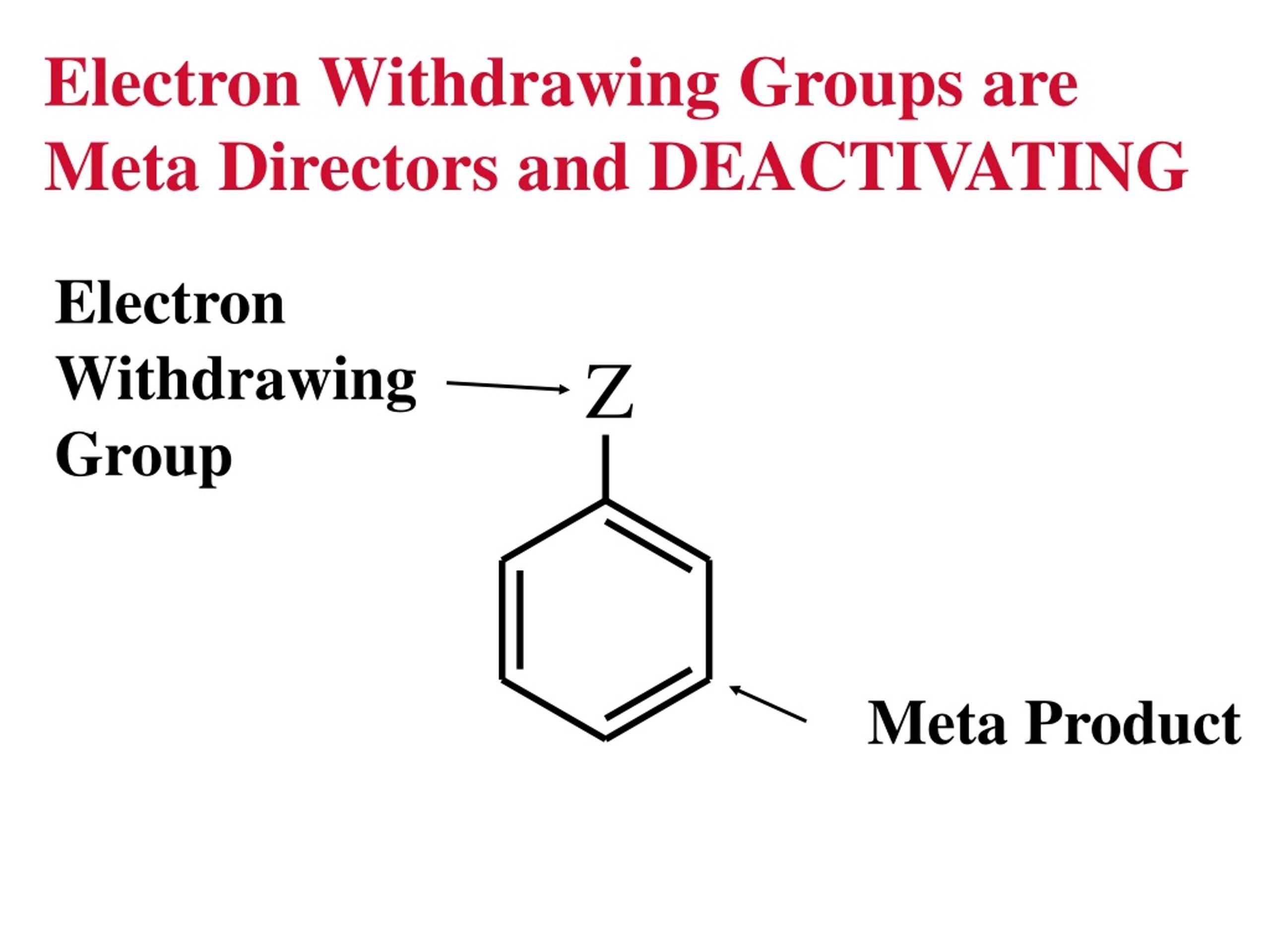
What Does Electron Withdrawing Group Mean . Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus.
from www.slideserve.com
Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus.
PPT Nitration of Methyl Benzoate PowerPoint Presentation, free download ID1264244
What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,.
From www.researchgate.net
Influence of an electronwithdrawing group (EWG) and cycle strain on... Download Scientific What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From byjus.com
Do electron withdrawing group increases reactivity towards nucleophilic addition reaction than What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.pinterest.com
صورة ذات صلة Organic chemistry, Organic chem, Chemistry What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From www.slideserve.com
PPT Nitration of Methyl Benzoate PowerPoint Presentation, free download ID1264244 What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From www.chemistrysteps.com
Nucleophilic Aromatic Substitution Chemistry Steps What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From www.scribd.com
Electron Withdrawing and Electron Donating Groups Functional Group Ion What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From kmsfert.blogspot.com
Electron Withdrawing Groups List / Effects of electron withdrawing group (EWG) and electron What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From quizlet.com
Electron Withdrawing Groups Diagram Quizlet What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.masterorganicchemistry.com
Acidity Trends In Organic Chemistry Master Organic Chemistry What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From www.semanticscholar.org
Table 1 from Enhancing effects of electronwithdrawing groups and metallic ions on halogen What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.slideserve.com
PPT Electrophilic Aromatic Substitution PowerPoint Presentation, free download ID6032354 What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From www.youtube.com
30.04 Electrondonating and withdrawing Groups YouTube What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.youtube.com
electronwithdrawing groups YouTube What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From brainly.in
Electron withdrawing and donating groups list Brainly.in What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From kmsfert.blogspot.com
Electron Withdrawing Groups List / Effects of electron withdrawing group (EWG) and electron What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.slideserve.com
PPT Electrophilic Aromatic Substitution PowerPoint Presentation ID227545 What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From www.clutchprep.com
Electron Withdrawing Groups Organic Chemistry Video Clutch Prep What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.numerade.com
SOLVED ElectronWithdrawing Groups in Carbonyls One other thing to consider about esters and What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From www.researchgate.net
Reactions using benzaldehyde with electron withdrawing groups and... Download Scientific Diagram What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.slideserve.com
PPT Chapter 2 Phenols PowerPoint Presentation, free download ID2962623 What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From forums.studentdoctor.net
Resonance Electron Withdrawing or Donating Student Doctor Network What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.youtube.com
What is Electron Withdrawing Groups ? Inductive effect & Acidity Basics One Chemistry What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From electronic--racket.blogspot.com
How To Identify Electron Withdrawing And Donating Groups What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From www.slideserve.com
PPT Chemistry of Aromatic Compounds PowerPoint Presentation, free download ID307370 What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From byjus.com
Why does electron withdrawing group increases the acidity and releasing groups decrease the What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.slideserve.com
PPT Chapter 2 Phenols PowerPoint Presentation, free download ID2962623 What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.youtube.com
Electron Withdrawing & Donating Groups Polarity YouTube What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From byjus.com
How does adding an electron withdrawing group to buy benzene diazonium salt an electron donating What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From www.slideserve.com
PPT Chapter 10 Chemical Bonding II PowerPoint Presentation, free download ID1866441 What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.slideserve.com
PPT Benzene and its Chemistry PowerPoint Presentation, free download ID1918503 What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From www.clutchprep.com
Electron Withdrawing Groups Organic Chemistry Video Clutch Prep What Does Electron Withdrawing Group Mean Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. The higher the electron density, the better the shield and thus the more protected the nucleus. What Does Electron Withdrawing Group Mean.
From www.researchgate.net
Substrate scope influence of an electronwithdrawing group at position... Download Scientific What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From forums.studentdoctor.net
Resonance Electron Withdrawing or Donating Student Doctor Network What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From byjus.com
47. List of electron withdrawing group and electron donating group and their priority order What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.
From www.youtube.com
Summary of electron.donating and withdrawing groups. Reactivity toward EAS YouTube What Does Electron Withdrawing Group Mean The higher the electron density, the better the shield and thus the more protected the nucleus. Electrophilic aromatic substitution can work when the alkene is in conjugation with an electron withdrawing group such as an ester,. What Does Electron Withdrawing Group Mean.