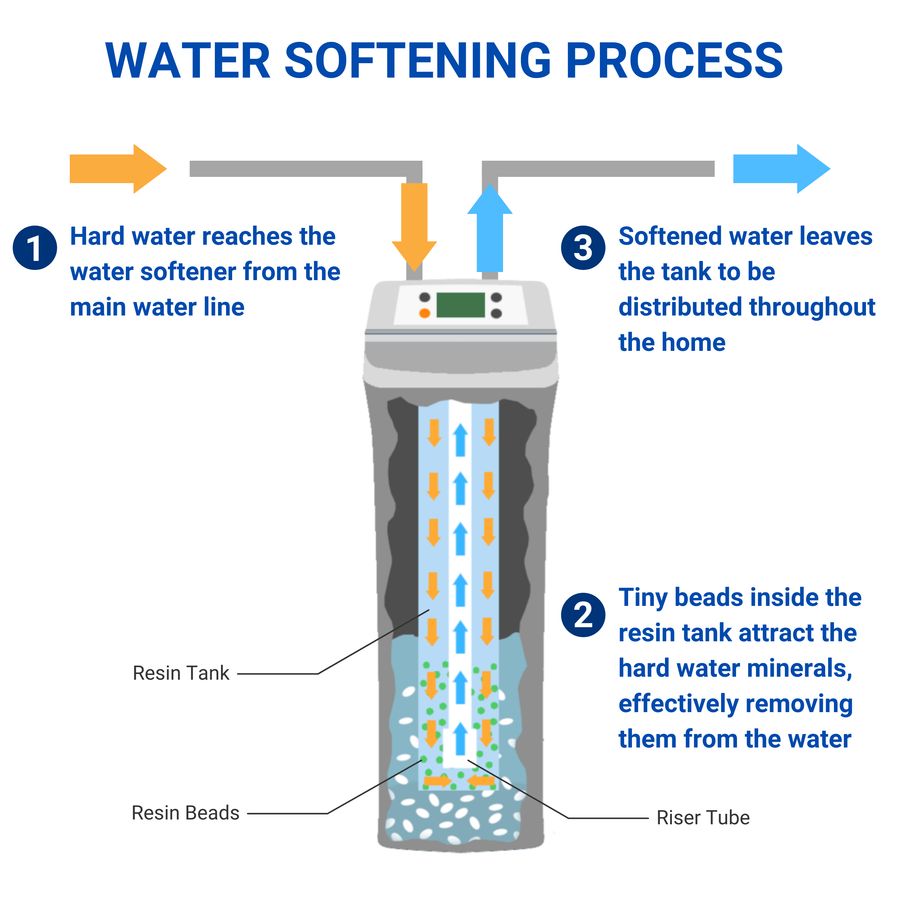
Water Softening Process . A water softener is a type of water treatment system that removes hardness minerals from a water supply. It is these ions in hard water that. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. The softeners that remove minerals use a process called ion exchange. This involves exchanging calcium and magnesium ions for sodium ions. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. How a water softener works depends on whether it removes minerals or changes the structure of minerals. Instead, it uses a process called chelation, which binds calcium.
from thehomesteadguide.com
This involves exchanging calcium and magnesium ions for sodium ions. Instead, it uses a process called chelation, which binds calcium. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. The softeners that remove minerals use a process called ion exchange. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. How a water softener works depends on whether it removes minerals or changes the structure of minerals. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. A water softener is a type of water treatment system that removes hardness minerals from a water supply. It is these ions in hard water that.
How Do Water Softeners Work? Simple StepByStep Process Explained
Water Softening Process This involves exchanging calcium and magnesium ions for sodium ions. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. How a water softener works depends on whether it removes minerals or changes the structure of minerals. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. The softeners that remove minerals use a process called ion exchange. Instead, it uses a process called chelation, which binds calcium. A water softener is a type of water treatment system that removes hardness minerals from a water supply. It is these ions in hard water that. This involves exchanging calcium and magnesium ions for sodium ions.
From autoctrls.com
The StepbyStep Process of a Water Softener Flow Diagram Explained Water Softening Process Instead, it uses a process called chelation, which binds calcium. A water softener is a type of water treatment system that removes hardness minerals from a water supply. This involves exchanging calcium and magnesium ions for sodium ions. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as. Water Softening Process.
From www.haguechicago.com
Water Softener Chicago A Guide To The Water Softening Process Water Softening Process Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Instead, it uses a process called chelation, which binds calcium. Water softening, the process of removing the dissolved calcium. Water Softening Process.
From www.indywaterpros.com
How Does a Water Softener Work? Water Softening Process Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. The softeners that remove minerals use a process called ion exchange. It is these ions in hard water that. How a water softener works depends on whether it removes minerals or changes the structure of minerals. This involves exchanging calcium and magnesium ions. Water Softening Process.
From www.thewatertreatments.com
WATER TREATMENTS SOFTENER Water Treatment Waste Water Treatment Water Softening Process Instead, it uses a process called chelation, which binds calcium. This involves exchanging calcium and magnesium ions for sodium ions. The softeners that remove minerals use a process called ion exchange. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. It is these ions in hard water that. How a water softener. Water Softening Process.
From netsolwater.com
How to Water Softening work with SodiumCycle Water Softening Process How a water softener works depends on whether it removes minerals or changes the structure of minerals. It is these ions in hard water that. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere. Water Softening Process.
From netsolwater.com
What are the components of a water softener under O and M Plan Water Softening Process How a water softener works depends on whether it removes minerals or changes the structure of minerals. Instead, it uses a process called chelation, which binds calcium. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. The softeners that remove minerals use a process called ion exchange. A water softener is a. Water Softening Process.
From www.water-rightgroup.com
How Water Softeners Work and Why You Need One WaterRight Water Softening Process Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. This involves exchanging calcium and magnesium ions for sodium ions. How a water softener works depends on whether it removes minerals or changes the structure of minerals. Instead, it uses a process called chelation, which binds calcium. A water softener is a type. Water Softening Process.
From thehomesteadguide.com
How Do Water Softeners Work? Simple StepByStep Process Explained Water Softening Process Instead, it uses a process called chelation, which binds calcium. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. How a water softener works depends on whether it removes. Water Softening Process.
From inspectapedia.com
Water Softener Regeneration Cycle Duration Fix a long water softener Water Softening Process Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. It is these. Water Softening Process.
From www.slideshare.net
Understanding hard water & water softening Water Softening Process Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. It is these ions in hard water that. Instead, it uses a process called chelation, which binds calcium. This involves exchanging calcium and magnesium ions for sodium ions. A water softener is a type of water treatment system that removes hardness minerals from. Water Softening Process.
From www.slideshare.net
Understanding hard water & water softening Water Softening Process Instead, it uses a process called chelation, which binds calcium. How a water softener works depends on whether it removes minerals or changes the structure of minerals. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. It is these ions in hard water that. The softeners. Water Softening Process.
From watersoftenerfacts.ca
How Softeners Work Water Softener Facts Water Softening Process Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. The softeners that remove minerals use a process called ion exchange. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening, the process of removing the dissolved. Water Softening Process.
From homewater101.com
How Does a Water Softener Work? Water Softening Process Diagram Water Softening Process A water softener is a type of water treatment system that removes hardness minerals from a water supply. This involves exchanging calcium and magnesium ions for sodium ions. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. It is these ions in hard water that. Water. Water Softening Process.
From watertechadvice.com
How Does a Water Softener Work? (What it is & What it Does Explained) Water Softening Process How a water softener works depends on whether it removes minerals or changes the structure of minerals. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. It is these ions in hard water that. Instead, it uses a process called chelation, which binds calcium. A water softener is a type of water. Water Softening Process.
From netsolwater.com
What is demineralization process of water softening Water Softening Process Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. A water softener is a type of water treatment system that removes hardness minerals from a water supply. How a water softener works depends on whether it removes minerals or changes the structure of minerals. Water softening,. Water Softening Process.
From upberi.com
How Does a Water Softener Work? + System Flow Diagram (2022) Water Softening Process How a water softener works depends on whether it removes minerals or changes the structure of minerals. The softeners that remove minerals use a process called ion exchange. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. A water softener is a type of water treatment. Water Softening Process.
From www.edrawmax.com
Water Softening Process Flow Diagram EdrawMax Templates Water Softening Process This involves exchanging calcium and magnesium ions for sodium ions. How a water softener works depends on whether it removes minerals or changes the structure of minerals. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. It is these ions in hard water that. Unlike hard water, softened water will not form. Water Softening Process.
From www.yakimawatersolutions.com
Learn How a Water Softener Works Yakima Water Solutions Water Softening Process Instead, it uses a process called chelation, which binds calcium. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. How a water softener works depends on whether it removes minerals or changes the structure. Water Softening Process.
From www.youtube.com
Zeolite process for water softening (Permutit process) Water Water Softening Process Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. How a water softener works depends on whether it removes minerals or changes the structure of minerals. This involves exchanging calcium and magnesium ions for sodium ions. Instead, it uses a process called chelation, which binds calcium. A water softener is a type. Water Softening Process.
From thehomesteadguide.com
How Do Water Softeners Work? Simple StepByStep Process Explained Water Softening Process It is these ions in hard water that. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. This involves exchanging calcium and magnesium ions for sodium ions. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. A water. Water Softening Process.
From sustainabilitymattersdaily.com
5 Methods of Water Softening Explained Water Softening Process The softeners that remove minerals use a process called ion exchange. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening is a process in which the. Water Softening Process.
From www.youtube.com
Water Softener Plant Operation I Softener water system I How does a Water Softening Process This involves exchanging calcium and magnesium ions for sodium ions. Instead, it uses a process called chelation, which binds calcium. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as. Water Softening Process.
From www.linkedin.com
What is the water softener system plant and how is it used? Water Softening Process Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. It is these ions in hard water that. A water softener is a type of water treatment system that removes. Water Softening Process.
From www.ecowater-softeners.co.uk
How Does A Water Softener Work? EcoWater Systems Water Softening Process Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. This involves exchanging calcium and magnesium ions for sodium ions. It is these ions in hard water that. The softeners that remove minerals use a process. Water Softening Process.
From homewater101.com
How Does a Water Softener Work? Water Softening Process Diagram Water Softening Process Instead, it uses a process called chelation, which binds calcium. It is these ions in hard water that. This involves exchanging calcium and magnesium ions for sodium ions. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Water softening, the process of removing the dissolved calcium and magnesium salts that cause. Water Softening Process.
From www.youtube.com
LEARN AND GROW !! ION EXCHANGE PROCESS FOR WATER SOFTENING ! YouTube Water Softening Process A water softener is a type of water treatment system that removes hardness minerals from a water supply. This involves exchanging calcium and magnesium ions for sodium ions. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. It is these ions in hard water that. The softeners that remove minerals use a. Water Softening Process.
From www.ecowater-softeners.co.uk
A Complete Guide To Choosing A Water Softener EcoWater Water Softening Process How a water softener works depends on whether it removes minerals or changes the structure of minerals. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. The softeners that. Water Softening Process.
From purewaterblog.com
The Water Softener Regeneration Cycle Explained! Water Treatment Water Softening Process This involves exchanging calcium and magnesium ions for sodium ions. It is these ions in hard water that. Instead, it uses a process called chelation, which binds calcium. Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. Water softening, the process of removing the dissolved calcium. Water Softening Process.
From colemanhanna.com
Water Treatment, Reverse Osmosis & Water Softeners Coleman Hanna Water Softening Process The softeners that remove minerals use a process called ion exchange. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. How a water softener works depends on whether it removes minerals or changes the structure of minerals. Water softening is a process in which the ions of calcium, magnesium and sometimes iron. Water Softening Process.
From waterfilterguru.com
How Does a Water Softener Work? + System Flow Diagram Water Softening Process Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. The softeners that. Water Softening Process.
From www.energy.gov
Purchasing and Maintaining A Water Softener Department of Energy Water Softening Process Unlike hard water, softened water will not form insoluble scale or precipitates in pipes and tanks or interfere with cleaners such as soap. Instead, it uses a process called chelation, which binds calcium. A water softener is a type of water treatment system that removes hardness minerals from a water supply. How a water softener works depends on whether it. Water Softening Process.
From betterwaterconcepts.com
How Does a Water Softener Work? Better Water Better Life Water Softening Process The softeners that remove minerals use a process called ion exchange. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. Instead, it uses a process called chelation, which binds calcium. Unlike hard water, softened water. Water Softening Process.
From waterfiltercast.com
How to Tell If Your Water Softener Is Working? (Easy Checklist) Water Softening Process This involves exchanging calcium and magnesium ions for sodium ions. Water softening is a process in which the ions of calcium, magnesium and sometimes iron are removed. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water. Instead, it uses a process called chelation, which binds calcium. The softeners that remove minerals use. Water Softening Process.
From www.korgentech.com
Water Softeners Water Softening Plants Water Softening Process A water softener is a type of water treatment system that removes hardness minerals from a water supply. How a water softener works depends on whether it removes minerals or changes the structure of minerals. It is these ions in hard water that. Water softening, the process of removing the dissolved calcium and magnesium salts that cause hardness in water.. Water Softening Process.
From sustainabilitymattersdaily.com
5 Methods of Water Softening Explained Water Softening Process Instead, it uses a process called chelation, which binds calcium. This involves exchanging calcium and magnesium ions for sodium ions. How a water softener works depends on whether it removes minerals or changes the structure of minerals. The softeners that remove minerals use a process called ion exchange. It is these ions in hard water that. Water softening, the process. Water Softening Process.