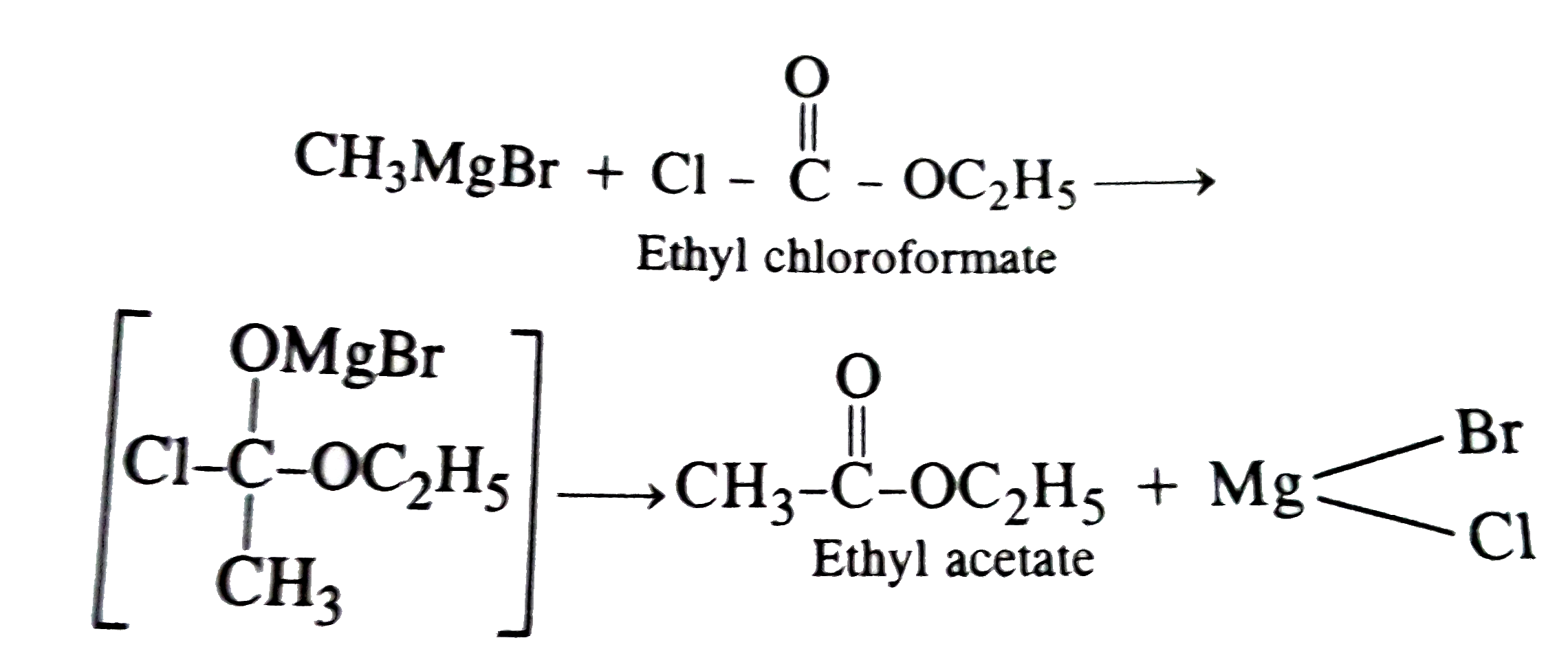Magnesium Iodide Answer . M g2+ +2i − →. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium metal forms a doubly charged cation. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. It forms various hydrates mgi2·xh2o. Make sense of the technique for the arrangement of magnesium iodide? the molecular or chemical formula of magnesium iodide is mgi 2. Magnesium iodide (mgi 2) can be. Iodide forms a singly charged anion.
from www.sarthaks.com
Iodide forms a singly charged anion. Make sense of the technique for the arrangement of magnesium iodide? the molecular or chemical formula of magnesium iodide is mgi 2. Magnesium metal forms a doubly charged cation. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. M g2+ +2i − →. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. It forms various hydrates mgi2·xh2o. Magnesium iodide (mgi 2) can be. magnesium iodide is an inorganic compound with the chemical formula mg i 2.
Ethyl acetate is obtained when methyl magnesium iodide reacts with
Magnesium Iodide Answer Make sense of the technique for the arrangement of magnesium iodide? magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. Magnesium metal forms a doubly charged cation. Iodide forms a singly charged anion. M g2+ +2i − →. magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium iodide (mgi 2) can be. It forms various hydrates mgi2·xh2o. the molecular or chemical formula of magnesium iodide is mgi 2. Make sense of the technique for the arrangement of magnesium iodide?
From molekula.com
Purchase Magnesium iodide anhydrous [10377589] online • Catalog Magnesium Iodide Answer Magnesium iodide (mgi 2) can be. the molecular or chemical formula of magnesium iodide is mgi 2. Iodide forms a singly charged anion. magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. Magnesium metal forms a doubly charged cation. M g2+ +2i − →. in magnesium iodide, the. Magnesium Iodide Answer.
From www.numerade.com
SOLVED A hydrate of magnesium iodide is heated tO remove the water Magnesium Iodide Answer magnesium iodide is an inorganic compound with the chemical formula mg i 2. the molecular or chemical formula of magnesium iodide is mgi 2. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Magnesium metal forms a doubly charged cation. Magnesium iodide (mgi 2) can be. Iodide forms a. Magnesium Iodide Answer.
From www.numerade.com
SOLVED Does reaction occur when aqueous solutions of iron(II) nitrate Magnesium Iodide Answer It forms various hydrates mgi2·xh2o. the molecular or chemical formula of magnesium iodide is mgi 2. Iodide forms a singly charged anion. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. Magnesium iodide (mgi 2) can be. magnesium iodide is a highly. Magnesium Iodide Answer.
From stock.adobe.com
Magnesium Iodide MgI2 molecule. Simple molecular formula consisting of Magnesium Iodide Answer It forms various hydrates mgi2·xh2o. Magnesium iodide (mgi 2) can be. M g2+ +2i − →. magnesium iodide is an inorganic compound with the chemical formula mg i 2. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. the molecular or chemical formula of magnesium iodide is mgi 2.. Magnesium Iodide Answer.
From www.doubtnut.com
[Odia] When nbutyl magnesium iodide is treated with water the product Magnesium Iodide Answer magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. Magnesium iodide (mgi 2) can be. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. Magnesium metal forms a doubly charged cation. M g2+ +2i. Magnesium Iodide Answer.
From www.meritnation.com
Acetone is obtained by hydrolysis of the addition product of methyl Magnesium Iodide Answer in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. It forms various hydrates mgi2·xh2o. M g2+ +2i − →. Iodide forms a singly charged anion. . Magnesium Iodide Answer.
From www.chegg.com
Solved 1) (CH3)SiCl, py 2) isopentyl magnesium iodide, ether Magnesium Iodide Answer Make sense of the technique for the arrangement of magnesium iodide? M g2+ +2i − →. magnesium iodide is an inorganic compound with the chemical formula mg i 2. Iodide forms a singly charged anion. It forms various hydrates mgi2·xh2o. the molecular or chemical formula of magnesium iodide is mgi 2. Magnesium metal forms a doubly charged cation.. Magnesium Iodide Answer.
From www.chegg.com
Solved What is the formula weight of magnesium iodide? How Magnesium Iodide Answer Magnesium iodide (mgi 2) can be. magnesium iodide is an inorganic compound with the chemical formula mg i 2. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon. Magnesium Iodide Answer.
From www.sarthaks.com
Ethyl acetate is obtained when methyl magnesium iodide reacts with Magnesium Iodide Answer magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. Magnesium metal forms a doubly charged cation. the molecular or chemical formula of magnesium iodide is mgi 2. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve. Magnesium Iodide Answer.
From brainly.in
what is the symbol of magnesium iodide Brainly.in Magnesium Iodide Answer magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. M g2+ +2i − →. Iodide forms a singly charged anion. Magnesium iodide (mgi 2) can be. the molecular or chemical formula. Magnesium Iodide Answer.
From slideplayer.com
Topic 1 Classification of Matter ppt download Magnesium Iodide Answer Iodide forms a singly charged anion. Magnesium metal forms a doubly charged cation. the molecular or chemical formula of magnesium iodide is mgi 2. magnesium iodide is an inorganic compound with the chemical formula mg i 2. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. in magnesium. Magnesium Iodide Answer.
From www.shutterstock.com
Magnesium Iodide Molecular Structure Formula Periodic Stock Vector Magnesium Iodide Answer Magnesium iodide (mgi 2) can be. Make sense of the technique for the arrangement of magnesium iodide? Magnesium metal forms a doubly charged cation. the molecular or chemical formula of magnesium iodide is mgi 2. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. in magnesium iodide, the magnesium. Magnesium Iodide Answer.
From www.sarthaks.com
Ethyl magnesium iodide reacts with propylamine to give Sarthaks Magnesium Iodide Answer Make sense of the technique for the arrangement of magnesium iodide? magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Magnesium metal forms a doubly charged cation. Iodide forms a singly charged anion. M g2+ +2i − →. It forms various hydrates mgi2·xh2o. in magnesium iodide, the magnesium ion (mg²⁺). Magnesium Iodide Answer.
From www.chegg.com
Solved 1) When 22.0 mL of a 6.65×104 M magnesium iodide Magnesium Iodide Answer M g2+ +2i − →. It forms various hydrates mgi2·xh2o. the molecular or chemical formula of magnesium iodide is mgi 2. Iodide forms a singly charged anion. magnesium iodide is an inorganic compound with the chemical formula mg i 2. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce.. Magnesium Iodide Answer.
From www.pw.live
Magnesium Iodide Formula, Structure And Properties Magnesium Iodide Answer Magnesium metal forms a doubly charged cation. magnesium iodide is an inorganic compound with the chemical formula mg i 2. Make sense of the technique for the arrangement of magnesium iodide? It forms various hydrates mgi2·xh2o. Magnesium iodide (mgi 2) can be. Iodide forms a singly charged anion. the molecular or chemical formula of magnesium iodide is mgi. Magnesium Iodide Answer.
From www.numerade.com
SOLVED In the laboratory you dissolve 21.9 g of magnesium iodide in a Magnesium Iodide Answer Magnesium iodide (mgi 2) can be. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. Magnesium metal forms a doubly charged cation. Iodide forms a singly charged anion. M g2+ +2i − →. the molecular or chemical formula of magnesium iodide is mgi. Magnesium Iodide Answer.
From www.bartleby.com
Answered The compound magnesium iodide, MgI2 is… bartleby Magnesium Iodide Answer Magnesium metal forms a doubly charged cation. It forms various hydrates mgi2·xh2o. Iodide forms a singly charged anion. Make sense of the technique for the arrangement of magnesium iodide? magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. magnesium iodide is an inorganic compound with the chemical formula mg i. Magnesium Iodide Answer.
From www.numerade.com
SOLVED Magnesium reacts with iodine gas at high temperatures to form Magnesium Iodide Answer Magnesium iodide (mgi 2) can be. M g2+ +2i − →. Magnesium metal forms a doubly charged cation. the molecular or chemical formula of magnesium iodide is mgi 2. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. Make sense of the technique. Magnesium Iodide Answer.
From www.pw.live
Magnesium Iodide Formula, Structure And Properties Magnesium Iodide Answer Make sense of the technique for the arrangement of magnesium iodide? Iodide forms a singly charged anion. It forms various hydrates mgi2·xh2o. magnesium iodide is an inorganic compound with the chemical formula mg i 2. the molecular or chemical formula of magnesium iodide is mgi 2. Magnesium metal forms a doubly charged cation. magnesium iodide is a. Magnesium Iodide Answer.
From kunduz.com
[ANSWERED] A 1 628g sample of a hydrate of Magnesium iodide Mgl xH2O Magnesium Iodide Answer magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium metal forms a doubly charged cation. Make sense of the technique for the arrangement of magnesium iodide? M g2+ +2i − →. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. in magnesium iodide, the. Magnesium Iodide Answer.
From www.numerade.com
SOLVED Which of the following compounds on reaction with ethyl Magnesium Iodide Answer in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. M g2+ +2i − →. Magnesium iodide (mgi 2) can be. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. the molecular or chemical formula. Magnesium Iodide Answer.
From www.youtube.com
Equation for MgI2 + H2O (Magnesium iodide + Water) YouTube Magnesium Iodide Answer Magnesium iodide (mgi 2) can be. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. the molecular or chemical formula of magnesium iodide is mgi 2. Iodide forms a singly charged anion. Magnesium metal forms a doubly charged cation. Make sense of the technique for the arrangement of magnesium iodide?. Magnesium Iodide Answer.
From www.chegg.com
Solved 1) (CH3) SiCI, py 2) isopentyl magnesium iodide, Magnesium Iodide Answer magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Iodide forms a singly charged anion. M g2+ +2i − →. Magnesium iodide (mgi 2) can be. magnesium iodide is an inorganic compound with the chemical formula mg i 2. in magnesium iodide, the magnesium ion (mg²⁺) has a positive. Magnesium Iodide Answer.
From www.numerade.com
SOLVED 'Magnesium reacts with iodine gas at high temperatures to form Magnesium Iodide Answer the molecular or chemical formula of magnesium iodide is mgi 2. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. magnesium iodide is an. Magnesium Iodide Answer.
From brainly.in
Which of the following compounds gives 2methyl propan2ol by the Magnesium Iodide Answer the molecular or chemical formula of magnesium iodide is mgi 2. M g2+ +2i − →. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. Magnesium metal forms a doubly charged cation. magnesium iodide is a highly stable compound under normal conditions,. Magnesium Iodide Answer.
From www.youtube.com
Draw the Lewis Structure of MgI2 (magnesium iodide) YouTube Magnesium Iodide Answer It forms various hydrates mgi2·xh2o. the molecular or chemical formula of magnesium iodide is mgi 2. magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium iodide (mgi 2) can be. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Magnesium metal forms a doubly. Magnesium Iodide Answer.
From stock.adobe.com
Magnesium iodide mgi2 molecule. Simple molecular formula consisting of Magnesium Iodide Answer Magnesium metal forms a doubly charged cation. the molecular or chemical formula of magnesium iodide is mgi 2. It forms various hydrates mgi2·xh2o. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Make sense of the technique for the arrangement of magnesium iodide? M g2+ +2i − →. Iodide forms. Magnesium Iodide Answer.
From brainly.com
The diagram shows how magnesium and iodine atoms form magnesium iodide Magnesium Iodide Answer Make sense of the technique for the arrangement of magnesium iodide? It forms various hydrates mgi2·xh2o. the molecular or chemical formula of magnesium iodide is mgi 2. Magnesium metal forms a doubly charged cation. magnesium iodide is an inorganic compound with the chemical formula mg i 2. in magnesium iodide, the magnesium ion (mg²⁺) has a positive. Magnesium Iodide Answer.
From www.vedantu.com
The reaction of methyl magnesium iodide with acetone followed by Magnesium Iodide Answer Iodide forms a singly charged anion. Make sense of the technique for the arrangement of magnesium iodide? It forms various hydrates mgi2·xh2o. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. the molecular or chemical formula of magnesium iodide is mgi 2. in magnesium iodide, the magnesium ion (mg²⁺). Magnesium Iodide Answer.
From www.chegg.com
Solved Magnesium reacts with iodine gas at high temperatures Magnesium Iodide Answer in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. Make sense of the technique for the arrangement of magnesium iodide? Iodide forms a singly charged anion. Magnesium iodide (mgi 2) can be. magnesium iodide is an inorganic compound with the chemical formula mg. Magnesium Iodide Answer.
From www.numerade.com
SOLVED In the electrolysis of aqueous magnesium iodide, there are two Magnesium Iodide Answer It forms various hydrates mgi2·xh2o. Magnesium metal forms a doubly charged cation. the molecular or chemical formula of magnesium iodide is mgi 2. Magnesium iodide (mgi 2) can be. M g2+ +2i − →. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Make sense of the technique for the. Magnesium Iodide Answer.
From guides.byjusweb.com
Magnesium Iodide Formula Definition, Concepts and Examples Magnesium Iodide Answer Make sense of the technique for the arrangement of magnesium iodide? Magnesium metal forms a doubly charged cation. It forms various hydrates mgi2·xh2o. the molecular or chemical formula of magnesium iodide is mgi 2. Magnesium iodide (mgi 2) can be. Iodide forms a singly charged anion. magnesium iodide is a highly stable compound under normal conditions, but it. Magnesium Iodide Answer.
From www.youtube.com
Preparation & Properties of Magnesium iodide YouTube Magnesium Iodide Answer M g2+ +2i − →. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two electrons to achieve a stable electron. Make sense of the technique for the arrangement of magnesium iodide? magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Magnesium. Magnesium Iodide Answer.
From www.doubtnut.com
Methyl magnesium iodide is converted to ethyl magnesium iodide Magnesium Iodide Answer magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. magnesium iodide is an inorganic compound with the chemical formula mg i 2. M g2+ +2i − →. Iodide forms a singly charged anion. the molecular or chemical formula of magnesium iodide is mgi 2. Magnesium iodide (mgi 2) can. Magnesium Iodide Answer.
From www.numerade.com
SOLVED Which one of the following compounds will react with methyl Magnesium Iodide Answer It forms various hydrates mgi2·xh2o. Magnesium iodide (mgi 2) can be. Magnesium metal forms a doubly charged cation. M g2+ +2i − →. Iodide forms a singly charged anion. the molecular or chemical formula of magnesium iodide is mgi 2. in magnesium iodide, the magnesium ion (mg²⁺) has a positive charge of +2, indicating the loss of two. Magnesium Iodide Answer.