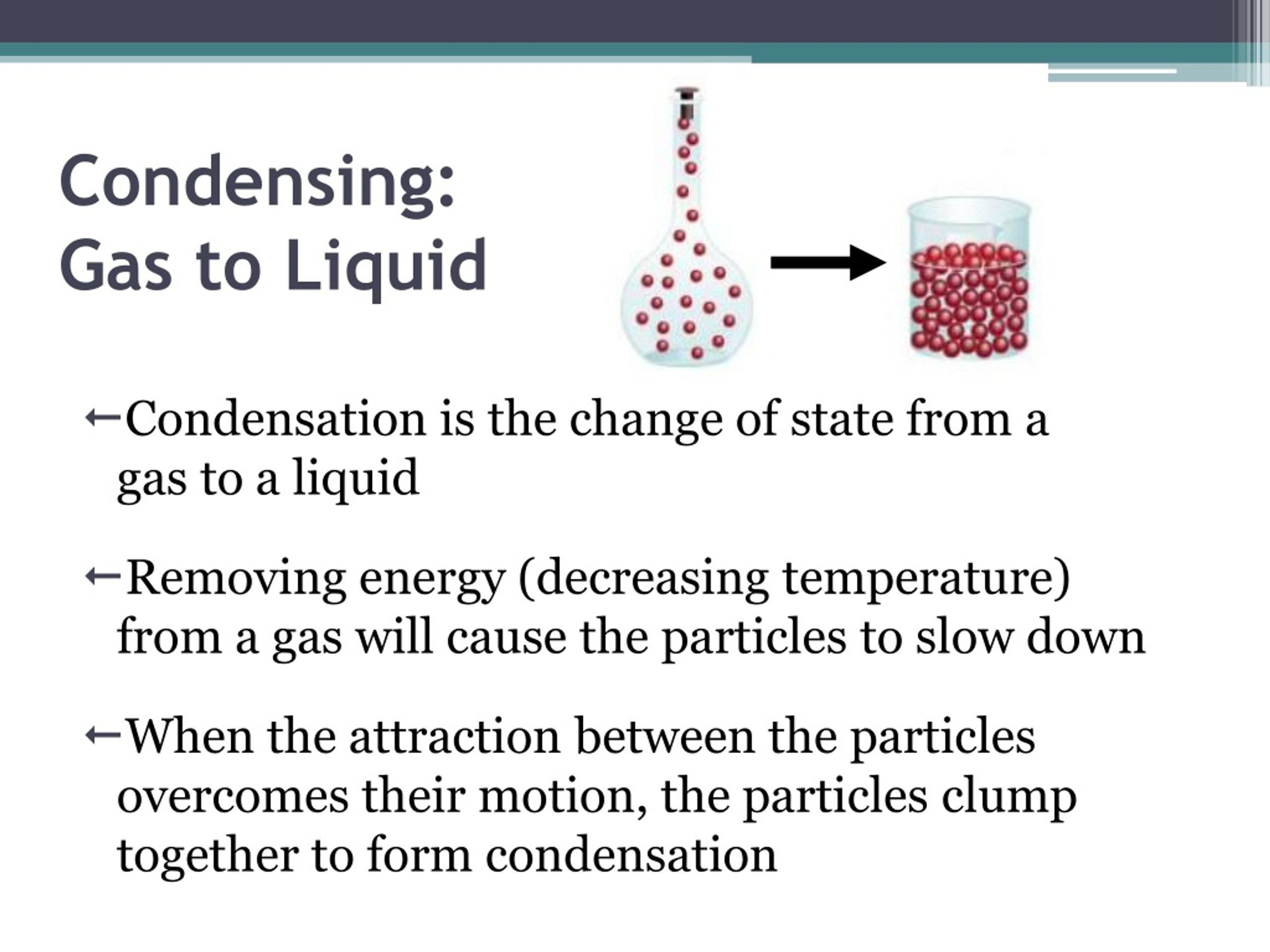
To Condense Gas To Liquid I Should Use . Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. condensation in liquid nitrogen production. So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. Evaporation is the conversion of a liquid to its vapor below the boiling. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. Nitrogen gas is commonly used in various industrial and scientific applications. when a gas cools down, it can change state back into a liquid. When gas molecules transfer their. as the altitude increases, the boiling point decreases.
from www.slideserve.com
So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. when a gas cools down, it can change state back into a liquid. Evaporation is the conversion of a liquid to its vapor below the boiling. as the altitude increases, the boiling point decreases. Nitrogen gas is commonly used in various industrial and scientific applications. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. condensation in liquid nitrogen production. condensation is the process in which molecules of a gas slow down, come together, and form a liquid.
PPT Essential Question How do particles behave in the four states of
To Condense Gas To Liquid I Should Use if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. Nitrogen gas is commonly used in various industrial and scientific applications. So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. as the altitude increases, the boiling point decreases. When gas molecules transfer their. condensation in liquid nitrogen production. Evaporation is the conversion of a liquid to its vapor below the boiling. when a gas cools down, it can change state back into a liquid. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the.
From exocprdpx.blob.core.windows.net
Solid Liquid And Gas Particles at Mui Jefferson blog To Condense Gas To Liquid I Should Use as the altitude increases, the boiling point decreases. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. When gas molecules transfer their. when a gas. To Condense Gas To Liquid I Should Use.
From slideplayer.com
Science Starter (Week 12, Day 2, 4/19/16) ppt download To Condense Gas To Liquid I Should Use as the altitude increases, the boiling point decreases. When gas molecules transfer their. Nitrogen gas is commonly used in various industrial and scientific applications. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. condensation is the process in which molecules of a. To Condense Gas To Liquid I Should Use.
From slideplayer.com
Vapor Pressure Vaporization change from liquid to gas at boiling To Condense Gas To Liquid I Should Use When gas molecules transfer their. as the altitude increases, the boiling point decreases. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. in fact, we know that at sufficiently low temperatures,. To Condense Gas To Liquid I Should Use.
From www.slideserve.com
PPT Chapter 1 Matter and Energy PowerPoint Presentation, free To Condense Gas To Liquid I Should Use So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at. To Condense Gas To Liquid I Should Use.
From www.worksheetsplanet.com
What is Condensation Definition of Condensation To Condense Gas To Liquid I Should Use Nitrogen gas is commonly used in various industrial and scientific applications. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. Evaporation is the conversion of a liquid. To Condense Gas To Liquid I Should Use.
From www.slideserve.com
PPT Aim What conditions would cause a gas to condense? PowerPoint To Condense Gas To Liquid I Should Use if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. Nitrogen gas is commonly used in various industrial and scientific applications. condensation in liquid nitrogen production. when a gas cools down, it can change state back into a liquid. Condensation changes the state. To Condense Gas To Liquid I Should Use.
From slideplayer.com
Molecular Theory Video ppt download To Condense Gas To Liquid I Should Use as the altitude increases, the boiling point decreases. condensation in liquid nitrogen production. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. when a gas cools down, it can change state back into a liquid. So when the warm steam hits the surface of, say, a cold. To Condense Gas To Liquid I Should Use.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock To Condense Gas To Liquid I Should Use Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. when a gas cools down, it can change state back into a liquid. condensation is the process in which molecules of a. To Condense Gas To Liquid I Should Use.
From www.youtube.com
Effect of pressure Change of state from gas to liquid CBSE Class 9 To Condense Gas To Liquid I Should Use when a gas cools down, it can change state back into a liquid. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. in. To Condense Gas To Liquid I Should Use.
From exocprdpx.blob.core.windows.net
Solid Liquid And Gas Particles at Mui Jefferson blog To Condense Gas To Liquid I Should Use So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. When gas molecules transfer their. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. condensation is the process in which molecules of a gas slow down, come together, and form a liquid.. To Condense Gas To Liquid I Should Use.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation, free To Condense Gas To Liquid I Should Use Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. condensation in liquid nitrogen production. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. when a gas cools down, it can change state back into. To Condense Gas To Liquid I Should Use.
From www.slideserve.com
PPT Essential Question How do particles behave in the four states of To Condense Gas To Liquid I Should Use Evaporation is the conversion of a liquid to its vapor below the boiling. So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. When gas molecules transfer their. if a substance. To Condense Gas To Liquid I Should Use.
From www.numerade.com
SOLVED A condenser is a device used to condense a gaseous substance To Condense Gas To Liquid I Should Use in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. Evaporation is the conversion of a liquid to its vapor below the boiling. as the altitude increases, the boiling point decreases. Nitrogen gas is commonly used in various industrial and scientific applications. When gas molecules transfer their. So when the. To Condense Gas To Liquid I Should Use.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps To Condense Gas To Liquid I Should Use Nitrogen gas is commonly used in various industrial and scientific applications. When gas molecules transfer their. So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. when a gas cools down, it can. To Condense Gas To Liquid I Should Use.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk To Condense Gas To Liquid I Should Use as the altitude increases, the boiling point decreases. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. condensation is the process in which molecules of a gas slow down, come together,. To Condense Gas To Liquid I Should Use.
From krystalminnicholson.blogspot.com
Which Best Describes the Transition From Gas to Liquid To Condense Gas To Liquid I Should Use as the altitude increases, the boiling point decreases. when a gas cools down, it can change state back into a liquid. When gas molecules transfer their. Evaporation is the conversion of a liquid to its vapor below the boiling. condensation is the process in which molecules of a gas slow down, come together, and form a liquid.. To Condense Gas To Liquid I Should Use.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation ID1115834 To Condense Gas To Liquid I Should Use if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. as the altitude increases, the boiling point decreases. When gas molecules transfer their. Nitrogen gas. To Condense Gas To Liquid I Should Use.
From www.youtube.com
CONDENSATION CHANGING OF MATTER (GAS LIQUID) SCIENCE 3 QUARTER 1 To Condense Gas To Liquid I Should Use condensation in liquid nitrogen production. when a gas cools down, it can change state back into a liquid. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. Nitrogen gas is commonly used in various industrial and scientific applications. When gas molecules transfer their. Condensation changes the state of. To Condense Gas To Liquid I Should Use.
From www.youtube.com
How Does Water Go From A Gas To A Liquid? Condensation YouTube To Condense Gas To Liquid I Should Use when a gas cools down, it can change state back into a liquid. When gas molecules transfer their. condensation in liquid nitrogen production. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. Evaporation is the conversion of a liquid to its vapor below the boiling. Nitrogen gas is. To Condense Gas To Liquid I Should Use.
From www.studocu.com
1 HOW TO CONDENSE GAS TO LIQUID Problem 1 Controller gain Kc was To Condense Gas To Liquid I Should Use condensation in liquid nitrogen production. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. condensation is the process in which molecules of a. To Condense Gas To Liquid I Should Use.
From dxoikfyih.blob.core.windows.net
Liquid Definition Chemistry at Stanley Schreiber blog To Condense Gas To Liquid I Should Use When gas molecules transfer their. as the altitude increases, the boiling point decreases. Nitrogen gas is commonly used in various industrial and scientific applications. condensation in liquid nitrogen production. when a gas cools down, it can change state back into a liquid. condensation is the process in which molecules of a gas slow down, come together,. To Condense Gas To Liquid I Should Use.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science To Condense Gas To Liquid I Should Use Nitrogen gas is commonly used in various industrial and scientific applications. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. condensation in liquid nitrogen production. When gas molecules transfer their. So when. To Condense Gas To Liquid I Should Use.
From exowbyyyo.blob.core.windows.net
Example Of Condensation And Evaporation at Bonnie Wilson blog To Condense Gas To Liquid I Should Use Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. as the altitude increases, the boiling point decreases. So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. if a substance is in a closed container at the boiling point, then the. To Condense Gas To Liquid I Should Use.
From slideplayer.com
Solids and Liquids Chapter 14 Chem B. ppt download To Condense Gas To Liquid I Should Use as the altitude increases, the boiling point decreases. So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. when a gas cools down, it can change state back into a liquid. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a. To Condense Gas To Liquid I Should Use.
From slideplayer.com
The Behavior of Gases. ppt download To Condense Gas To Liquid I Should Use when a gas cools down, it can change state back into a liquid. condensation in liquid nitrogen production. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. Nitrogen gas is commonly used in various industrial and scientific applications. condensation is the. To Condense Gas To Liquid I Should Use.
From www.slideserve.com
PPT Molecular Theory States of Matter Phase Changes To Condense Gas To Liquid I Should Use When gas molecules transfer their. when a gas cools down, it can change state back into a liquid. condensation in liquid nitrogen production. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. Nitrogen gas is commonly used in various industrial and scientific applications. as the altitude increases, the boiling. To Condense Gas To Liquid I Should Use.
From exohzkqeb.blob.core.windows.net
Do Gases Condense To Form Liquids at Yolanda Cortez blog To Condense Gas To Liquid I Should Use So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. Nitrogen gas is commonly used in various industrial and scientific applications. condensation is the process. To Condense Gas To Liquid I Should Use.
From slideplayer.com
Heat Phase Changes LabRat Scientific © ppt download To Condense Gas To Liquid I Should Use as the altitude increases, the boiling point decreases. condensation in liquid nitrogen production. Nitrogen gas is commonly used in various industrial and scientific applications. When gas molecules transfer their. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. Condensation changes the state of matter from gaseous to liquid,. To Condense Gas To Liquid I Should Use.
From www.slideserve.com
PPT Chapter 2 “ Matter and Change” p. 38 PowerPoint Presentation ID To Condense Gas To Liquid I Should Use when a gas cools down, it can change state back into a liquid. When gas molecules transfer their. condensation is the process in which molecules of a gas slow down, come together, and form a liquid. Evaporation is the conversion of a liquid to its vapor below the boiling. Condensation changes the state of matter from gaseous to. To Condense Gas To Liquid I Should Use.
From www.slideserve.com
PPT Physical Science Unit 3 PowerPoint Presentation, free download To Condense Gas To Liquid I Should Use Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. as the altitude increases, the boiling point decreases. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the. So when the warm steam hits the surface of,. To Condense Gas To Liquid I Should Use.
From slideplayer.com
Liquids, Solids and Changes of State ppt video online download To Condense Gas To Liquid I Should Use condensation is the process in which molecules of a gas slow down, come together, and form a liquid. condensation in liquid nitrogen production. when a gas cools down, it can change state back into a liquid. Nitrogen gas is commonly used in various industrial and scientific applications. Condensation changes the state of matter from gaseous to liquid,. To Condense Gas To Liquid I Should Use.
From www.youtube.com
Gas to liquids Process YouTube To Condense Gas To Liquid I Should Use as the altitude increases, the boiling point decreases. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. Evaporation is the conversion of a liquid to its vapor below the boiling.. To Condense Gas To Liquid I Should Use.
From www.slideserve.com
PPT Chemistry…. PowerPoint Presentation, free download ID1113620 To Condense Gas To Liquid I Should Use When gas molecules transfer their. when a gas cools down, it can change state back into a liquid. So when the warm steam hits the surface of, say, a cold mirror it turns back into water again. in fact, we know that at sufficiently low temperatures, any real gas, when compressed, must undergo a transition. Condensation changes the. To Condense Gas To Liquid I Should Use.
From www.snexplores.org
Explainer What are the different states of matter? To Condense Gas To Liquid I Should Use condensation is the process in which molecules of a gas slow down, come together, and form a liquid. When gas molecules transfer their. when a gas cools down, it can change state back into a liquid. Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. if a substance is. To Condense Gas To Liquid I Should Use.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State To Condense Gas To Liquid I Should Use Condensation changes the state of matter from gaseous to liquid, often accompanied by a decrease in volume. When gas molecules transfer their. when a gas cools down, it can change state back into a liquid. if a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at. To Condense Gas To Liquid I Should Use.